Abstract
Etv2 (Ets Variant 2) has been shown to be an indispensable gene for the development of hematopoietic cells (HPCs)/endothelial cells (ECs). However, how Etv2 specifies the mesoderm-generating HPCs/ECs remains incompletely understood. In embryonic stem cell (ESC) differentiation culture and Etv2-null embryos, we show that Etv2 is dispensable for generating primitive Flk-1+/PDGFRα+ mesoderm but is required for the progression of Flk-1+/PDGFRα+ cells into vascular/hematopoietic mesoderm. Etv2-null ESCs and embryonic cells were arrested as Flk-1+/PDGFRα+ and failed to generate Flk-1+/PDGFRα− mesoderm. Flk-1+/Etv2+ early embryonic cells showed significantly higher hemato-endothelial potential than the Flk-1+/Etv2− population, suggesting that Etv2 specifies a hemato-endothelial subset of Flk-1+ mesoderm. Critical hemato-endothelial genes were severely down-regulated in Etv2-null Flk-1+ cells. Among those genes Scl, Fli1, and GATA2 were expressed simultaneously with Etv2 in early embryos and seemed to be critical targets. Etv2 reexpression in Etv2-null cells restored the development of CD41+, CD45+, and VE-cadherin+ cells. Expression of Scl or Fli1 alone could also restore HPCs/ECs in the Etv2-null background, indicating that these 2 genes are critical downstream targets. Furthermore, VEGF induced Etv2 potently and rapidly in Flk-1+ mesoderm. We propose that Flk-1+/PDGFRα+ primitive mesoderm is committed into Flk-1+/PDGFRα− vascular mesoderm through Etv2 and that up-regulation of Etv2 by VEGF promotes this commitment.
Introduction
Based on observations that several single-gene mutations severely affect both endothelial cell (EC) and hematopoietic cell (HPC) lineages, ECs and HPCs are proposed to originate from common precursors called hemangioblasts.1,2 In zebrafish, cloche is the most classic mutant affecting both ECs and HPCs.3,4 Deleting Flk-1, a major signaling receptor for VEGF, in mice causes defects in both ECs and HPCs.5,6 However, in vitro differentiation of Flk-1–null embryonic stem cells (ESCs) generates both ECs and HPCs, albeit at much lower efficiencies.7,8 These findings demonstrate that Flk-1 is dispensable for the differentiation of hemato-endothelial precursors but is required for the proliferation of these progenitor cells.
Etv2 (Ets variant 2), identified in zebrafish as etsrp71, has been reported to be a key molecule for investigating EC and HPC development.9-11 In etsrp71 mutants, vasculogenesis and myelopoiesis are affected, leaving primitive erythropoiesis relatively intact. Conversely, overexpression of etsrp71 induces ectopic EC generation indicating a strong vasculogenic potential of etsrp71.12 In mice, Etv-2 disruption results in a lack of Flk-1 expression, leading to the total loss of ECs and HPCs.10,13 In ESCs, Etv2 overexpression up-regulates Flk-1 and subsequent EC generation.10 From these results, Etv2 was hypothesized to be induced by bone morphogenic protein (BMP), Notch, and Wnt signaling and then transactivate Flk1. However, the phenotype discrepancy between the etsrp71 zebrafish mutant and Etv2-knockout mice raised the possibility that Etv2 may have more complex functions than proposed as an activator of Flk-1. Recent studies in zebrafish showed that, in addition to Flk-1, EC or HPC genes can also be up-regulated more robustly by Etv2.14,15 Using Etv2 Venus knock-in mice and Etv2-null ESC lines (Etv2-Venus+/− and Etv2−/−, respectively), the role of Etv2 and downstream target genes in EC and HPC development from mesoderm were investigated in the present study.
Methods
Etv2-Venus ESCs and mice
Targeting vector for the mouse Etv-2 locus was constructed using the Gateway cassette vector system containing Venus and Cre removable Neo resistance cassette.16 Genomic sequences corresponding to 111658-119169 (accession number 167978.4) on the 5′side and 119172-122639 (accession number 167978.4) on the 3′ side were used as homology arms for recombination, and Venus was subcloned in-frame into the endogenous Etv2 allele. The resulting targeting construct and TT2 ESCs were used to generate Etv2-Venus knock-in mice (CDB0675K). Original and modified Etv2 loci are depicted in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Etv2 locus on the other allele was targeted to generate homozygous Etv2-null ESCs. These Etv2-Venus mice or gene-trap line 141H7 were used to generate Etv2-null embryos.13
RNA isolation and expression profiling
RNA was purified using the RNeasy MinElute column (QIAGEN) and reverse transcribed by Revatrace (TOYOBO). Real-time PCR was performed with the PowerSyber Green kit (ABI) using ABI 9700. Microarray analysis was performed using Mouse Genome 430 2.0 Array (Affymetrix) using 2 differentiation cultures that were started independently. Oligonucleotide primers for quantitative PCR (Q-PCR) are listed in supplemental Table 3. All microarray data are available in the Gene Expression Omnibus (GEO) under accession numbers GSE31743 and GSE31744.
Cell culture
ESCs were differentiated on OP9 cells as described previously.17 Briefly, 105 ESCs/25 cm2 were seeded onto OP9. To induce Etv2-Venus+ cells BMP4, VEGF, and FGF8b were added from day 3 of differentiation. For HPC differentiation, SCF (100 ng/mL), G-CSF, (10 ng/mL), IL-3 (5 U/mL), erythropoietin (5 ng/mL), and VEGF (20 ng/mL) were added. Abs used for flow cytometry were Flk-1 (AVAS12), PDGFRα (APA5), CD41 (MWReg30), ICAM2 (3C4), CD31 (Mec13.3), CD90.1 (Thy1.1; eBioscience), and VE-cadherin (VECAD1; BioLegend). For transgene rescue in Etv2-null cells Etv2, Scl, Fli1, or GATA2 were inserted into the piggyBac (PB)–based expression cassette under the control of the Tetracyclin (Tet)–responsive promoter (Guo et al18 and modified by H. Niwa). Transgene expression was monitored by IRES-tagged CD90.1 staining. These plasmids were cotransfected with PB-CAG-tTA-IRESNeo and PBase expression plasmids (gifts from H. Niwa and Guo et al) using X-fect (Clontech) to obtain Tet-responsive clones. Those cells established as undifferentiated ESC clones in the presence of Tet were differentiated on OP9 without Tet. On differentiation day 3, transgene-expressing cells were sorted by CD90.1, further differentiated on OP9 until day 10, and stained with the indicated Abs. Serum-free culture of Flk-1+ mesoderm was performed as described previously.19
Immunostaining
Etv2 expression in Etv2-Venus mice was detected using rabbit or chicken anti-green fluorescent protein (anti-GFP; Invitrogen; 1:1000) Abs. Whole-mount immunostaining was performed using Abs against Flk-1 (BD Biosciences or R&D Systems), PDGFRα (Cell Signaling Technology), and GATA1 (N6; Santa Cruz Biotechnology).
In situ hybridization
Whole-mount in situ hybridization was performed essentially as described previously.20 Probes for each gene were synthesized using the sequences as follows with the DIG RNA labeling kit. (Roche): GATA2 1589-2059, accession number NM008090; Fli1 1284-1543, accession number NM008026; Scl 860-1428, accession number M59764; and Runx1 2048-2518, accession number NM009821.
EMSA
For the electrophoretic mobility shift assay (EMSA), Etv2 protein was synthesized from pCS2-Etv2 with the TNT in vitro translation kit (Promega). Reticulocyte lysate containing translated Etv2 was incubated with labeled probes with or without competing oligonucleotides (supplemental Table 3) in binding buffer. Electrophoretic mobility shift was analyzed by electrophoresis in 0.5× TBE gel and subsequent autoradiography.
ChIP
Mouse EC line F2 or CCE ESCs were transfected with HA-tagged Etv2 and ChIP analysis was performed using the ChIP-IT kit (Active Motif), anti-HA antibody (12CA5 or 3F10; Roche), and the indicated primer sets (supplemental Table 3). Flk-1+ cells from HA-Etv2 CCE cells were sorted after differentiation for 4 days.
Luciferase assay
Indicated sequences of the Scl, Fli1, and GATA2 genomes were PCR amplified using KOD-FX polymerase (TOYOBO) and ligated to pGL3-SV40 vector (Promega): Scl, 15199029-15199673 (NT_039264.6); Fli1, 19119650-19120142 (NT_039472.7); and GATA2, 40461620-40463619 (NT_039353.7). Bovine aortic ECs were transfected with luciferase reporter vectors (wild-type and Etv2-binding sites mutated) and control, Etv2, Ets1, or Ets2 expression vectors using Lipofectamine 2000 (Invitrogen). Transfection efficiency was normalized with the pRLCMV-Renilla expression vector. Luciferase activity was measured according to the manufacturer's directions (Promega).
Microscope models for images
Whole-mount embryo samples in PBS were observed in an M205C microscope (Leica Microsystems). Fluorescent images were made with Prolong-Gold medium (Invitrogen) and BZ-9000 (Keyence). Figure 1Bd-e are images made using Entellan (Merck) and visualized on a DMI 6000B microscope (Leica Microsystems). Figure 2Aa shows bright images covered by PBS and imaged using a DMI4000B microscope (Leica Microsystems).
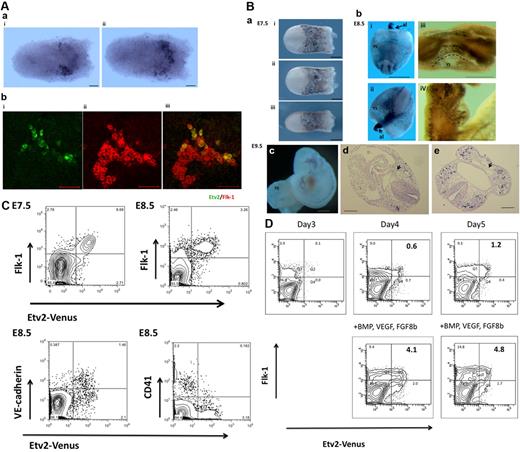
Etv2 expression in developing mouse embryos. (Aa) Etv2 is first detected in the posterolateral mesoderm around E7.0; panel i is the anterior and panel ii the posterior view. Scale bar indicates 50 μm. (b) Double immunostaining with anti-Flk-1 antibody revealed that Etv2 is expressed in a subset of Flk-1+ cells at this stage. Magnified view of the posterolateral mesoderm where Flk-1+ cells exists: Etv2-Green (i), Flk-1-Red (ii), and merged (iii). Scale bar indicates 50 μm. (Ba) Etv2 is expressed at E7.5 in the extra-embryonic mesoderm. Etv2 is almost colocalized with Flk-1 at this stage: (i) anterior view, (ii) posterior view, and (iii) lateral view. Scale bar indicates 200 μm. (b) At E8.5 expression was detected along the dorsal aorta, endocardium and allantois (al). Expression in the extra-embryonic yolk sac (YS) region was rapidly down-regulated. Scale bar indicates 500 μm. (c) Expression of Etv2 at E9.5. (d) Expression was detected along the vasculature. (e) Etv2 was barely detected in matured ECs incorporated into the vasculature (arrows). Scale bars in panels b and c indicate 500 μm; scale bars in panels d and e indicate 200 μm. (C) Flow cytometric analysis of Etv2 expression in developing embryos. (a) Flow cytometry of E7.5 and 8.5 Etv2-Venus+/− embryos. At E7.5, 76% of Flk-1+ cells were Etv2+. (b) At E8.5, Etv-2 expression was still restricted in the Flk-1+ population, with 56% of the Flk-1+ cells being Etv2-Venus+. (c) At E8.5, 79% of the VE-cadherin+ cells retained the Etv2 expression. Approximately 92% of the CD41+ HPCs were Etv2-Venus−, indicating the rapid down-regulation of Etv2 on HPC differentiation. (D) Etv2 expression in differentiating ESCs. Etv2-Venus ESCs were differentiated on OP9 cells. Mesoderm-inducing factors BMP4, FGF8b, and VEGF were added on day 3 of differentiation. On day 3, when Flk-1+ cells started to be seen, only 0.1% were Flk-1+/Etv2-Venus+ whereas 3.9% were Flk-1+/Etv2-Venus−. The addition of mesoderm-inducing factors promoted the generation of Etv2+ cells in 24 hours on day 4 (Flk-1+/Etv2-Venus+ cells from 0.6%-4.1%) and day 5 (Flk-1+/Etv2-Venus+ cells from 1.2%-4.8%). The majority of Etv2-Venus+ cells are present as Flk-1+.
Etv2 expression in developing mouse embryos. (Aa) Etv2 is first detected in the posterolateral mesoderm around E7.0; panel i is the anterior and panel ii the posterior view. Scale bar indicates 50 μm. (b) Double immunostaining with anti-Flk-1 antibody revealed that Etv2 is expressed in a subset of Flk-1+ cells at this stage. Magnified view of the posterolateral mesoderm where Flk-1+ cells exists: Etv2-Green (i), Flk-1-Red (ii), and merged (iii). Scale bar indicates 50 μm. (Ba) Etv2 is expressed at E7.5 in the extra-embryonic mesoderm. Etv2 is almost colocalized with Flk-1 at this stage: (i) anterior view, (ii) posterior view, and (iii) lateral view. Scale bar indicates 200 μm. (b) At E8.5 expression was detected along the dorsal aorta, endocardium and allantois (al). Expression in the extra-embryonic yolk sac (YS) region was rapidly down-regulated. Scale bar indicates 500 μm. (c) Expression of Etv2 at E9.5. (d) Expression was detected along the vasculature. (e) Etv2 was barely detected in matured ECs incorporated into the vasculature (arrows). Scale bars in panels b and c indicate 500 μm; scale bars in panels d and e indicate 200 μm. (C) Flow cytometric analysis of Etv2 expression in developing embryos. (a) Flow cytometry of E7.5 and 8.5 Etv2-Venus+/− embryos. At E7.5, 76% of Flk-1+ cells were Etv2+. (b) At E8.5, Etv-2 expression was still restricted in the Flk-1+ population, with 56% of the Flk-1+ cells being Etv2-Venus+. (c) At E8.5, 79% of the VE-cadherin+ cells retained the Etv2 expression. Approximately 92% of the CD41+ HPCs were Etv2-Venus−, indicating the rapid down-regulation of Etv2 on HPC differentiation. (D) Etv2 expression in differentiating ESCs. Etv2-Venus ESCs were differentiated on OP9 cells. Mesoderm-inducing factors BMP4, FGF8b, and VEGF were added on day 3 of differentiation. On day 3, when Flk-1+ cells started to be seen, only 0.1% were Flk-1+/Etv2-Venus+ whereas 3.9% were Flk-1+/Etv2-Venus−. The addition of mesoderm-inducing factors promoted the generation of Etv2+ cells in 24 hours on day 4 (Flk-1+/Etv2-Venus+ cells from 0.6%-4.1%) and day 5 (Flk-1+/Etv2-Venus+ cells from 1.2%-4.8%). The majority of Etv2-Venus+ cells are present as Flk-1+.
Hemato- endothelial potential in Etv2+/Flt-1+. Flk-1+/Etv2− or Flk-1+/Etv2+ cells from Etv2-Venus+/− embryos (E7.5 embryo; 500 cells/35-mm well, E8.5 embryo proper; 8000 cells/35-mm well) were cultured on OP9 for 10 days with growth factors (erythropoietin, IL-3, G-CSF, SCF, and VEGF). (Aa) HPCs were preferentially observed in E7.5 Flk-1+/Etv2+ population (bottom). Similar cells did not develop from Flk-1+/Etv2− cells (top). CD45+ cells or Flk-1+ plexus-like structures were only observed from Flk-1+/Etv2+ culture. Scale bar indicates 100 μm. (b) Flow cytometric analysis of cells generated from Flk-1+/Etv2− or Flk-1+/Etv2+ embryonic cells. Mac-1+CD45+granulocytes or Ter119+ erythroid cells developed from E7.5 Flk-1+/Etv2+ fraction. Almost no cells were seen in the same FSC/SSC area containing HPCs from Flk-1+/Etv2− cells cultured in the same condition. (Ba) Flk-1+/Etv2− or Flk-1+/Etv2+ cells from E8.5 intra-embryo parts also showed similar results. After 10 days of culture, CD45+HPCs or Flk-1+/ICAM2+ ECs were seen from Flk-1+/Etv2+ cells (bottom), whereas few or no similar cells developed from Flk-1+/Etv2− (top). Scale bar indicates 100 μm. (b) ECs developed more efficiently from E8.5 embryo proper Flk-1+/Etv2+ cells than from Flk-1+/Etv2− cells. CD45+c-Kit+cells were seen only from E8.5 embryo proper Flk-1+/Etv2+ population.
Hemato- endothelial potential in Etv2+/Flt-1+. Flk-1+/Etv2− or Flk-1+/Etv2+ cells from Etv2-Venus+/− embryos (E7.5 embryo; 500 cells/35-mm well, E8.5 embryo proper; 8000 cells/35-mm well) were cultured on OP9 for 10 days with growth factors (erythropoietin, IL-3, G-CSF, SCF, and VEGF). (Aa) HPCs were preferentially observed in E7.5 Flk-1+/Etv2+ population (bottom). Similar cells did not develop from Flk-1+/Etv2− cells (top). CD45+ cells or Flk-1+ plexus-like structures were only observed from Flk-1+/Etv2+ culture. Scale bar indicates 100 μm. (b) Flow cytometric analysis of cells generated from Flk-1+/Etv2− or Flk-1+/Etv2+ embryonic cells. Mac-1+CD45+granulocytes or Ter119+ erythroid cells developed from E7.5 Flk-1+/Etv2+ fraction. Almost no cells were seen in the same FSC/SSC area containing HPCs from Flk-1+/Etv2− cells cultured in the same condition. (Ba) Flk-1+/Etv2− or Flk-1+/Etv2+ cells from E8.5 intra-embryo parts also showed similar results. After 10 days of culture, CD45+HPCs or Flk-1+/ICAM2+ ECs were seen from Flk-1+/Etv2+ cells (bottom), whereas few or no similar cells developed from Flk-1+/Etv2− (top). Scale bar indicates 100 μm. (b) ECs developed more efficiently from E8.5 embryo proper Flk-1+/Etv2+ cells than from Flk-1+/Etv2− cells. CD45+c-Kit+cells were seen only from E8.5 embryo proper Flk-1+/Etv2+ population.
Results
Etv-2 is expressed in the Flk-1+ cell population in developing embryos and differentiating ESCs
Previous in situ hybridization showed Etv2 expression in the primitive streak mesoderm, including the blood island, and in developing ECs.10 To visualize Etv2 expression in detail, Etv2-Venus mice and differentiated ESCs were analyzed by anti-GFP immunostaining and flow cytometry. Whereas Etv2 expressions at embryonic day 8.5 (E8.5) or E9.5 by anti-GFP immunostaining were consistent with the previous in situ hybridization report, in the E7.0-E7.5 embryos, the expression pattern was different. Expression of Etv-2 began to appear around E7.0 within the Flk-1+ cells in the posterior mesoderm (Figure 1Aai-ii). At this stage, Etv2+ cells were seen in a subset of Flk-1+ cells (Figure 1Abi-iii). At E7.5, expression was detected in the extra-embryonic mesoderm (Figure 1Ba). Double staining with Flk-1 showed that most of the extra-embryonic Flk-1+ cells were Venus+, indicating the pivotal role of Flk-1 in generating endothelial and hematopoietic precursors at this stage (supplemental Figure 2A). At E8.5, expression was seen from the anterior to posterior part of the dorsal aorta, and in the endocardium and head mesenchyme region (Figure 1Bb and supplemental Figure 2B-C). However, little or no staining was observed in the extra-embryonic yolk sac area (Figure 1Bbi-iii and supplemental Figure 2B). At E9.5, expression was seen in developing vasculature mainly in the cardinal vein and scattered cells in cranial mesenchyme, but was undetectable in the yolk sac (Figure 1Bc). At E9.5, Etv2 expression was barely seen in the EC layer of mature vasculature except in the endocardium, indicating its rapid down-regulation in fully differentiated ECs (Figure 1Bd-e).
To understand the relationship between the expressions of Etv2 and other HPC/EC markers, Etv2-Venus+/− embryos were analyzed by flow cytometry using several Abs (Figure 1C). Both at E7.5 and E8.5, the majority of Etv2+ (Venus+) cells were present as Flk-1+ cells. At E7.5, Etv2 and Flk-1 were coexpressed in the majority of Flk-1+ cells (76%). At E8.5, Etv2 expression was still seen in > 70% of Flk-1+ cells. Whereas approximately 80% of VE-cadherin+ cells expressed Etv2+, < 10% of CD41+ cells expressed Etv2, indicating that Etv2 is down-regulated in cells fated to a primitive HPC lineage. These findings suggest that Etv2 expression is transient in endothelial and hematopoietic precursors and that it rapidly declines once cells are fully differentiated.
We also analyzed the expression of Etv2 in differentiating Etv2-Venus ESCs on OP9 (Figure 1D). At day 2.5-3 of differentiation we started to see Flk-1+ cells before the appearance of significant numbers of Venus+ cells. The proportion of Etv2+ (Venus+) cells leveled off at day 4 or 5 of differentiation, followed by significant reduction after day 6 of differentiation, reflecting the similar pattern of transient Etv2 expression in embryonic development. Etv2 expression in ESC differentiation was also evident only within the Flk-1+ cell population. As seen in the earliest stage of Etv2 expression in embryos, only a fraction of Flk-1+ cells were Etv2+ (Venus+) when Etv2 expression began at day 2.5-3. These observations suggest that Etv2 expression represents a subset of Flk-1+ cells being committed to ECs or HPCs.
HPC/EC precursors are preferentially localized in the Etv2+ population in early embryos
To investigate whether Etv2+ cells are HPC/EC precursors within Flk-1+ cells, the Flk-1+Etv2+ or Flk-1+ Etv2− population was sorted from E7.5 or E8.5 embryos and cultured on OP9. E7.5 and embryo proper parts of E8.5 embryos were analyzed because Etv2+ populations in these parts are the cells that start to express Etv2. At later stages, some Etv2− cells might have been committed to HPCs/ECs by prior transient expression of Etv2. After culturing for 10 days, HPCs/ECs were observed only from Flk-1+Etv2+ fractions in E7.5 embryos, whereas few HPCs/ECs developed from Flk-1+Etv2−, indicating that HPC/EC precursors are preferentially localized in Flk-1+Etv2+ populations (Figure 2Aa-b). From the embryo proper parts of E8.5 embryos, ECs were generated from both the Flk-1+Etv2+ and the Flk-1+Etv2− population, however, with much less efficiency from Flk-1+Etv2−. CD45+ cells were preferentially observed from the Flk-1+Etv2+ population (Figure 2Ba-b). The finding that Etv2+Flk-1+cells could generate CD45+ HPCs or ECs more efficiently than Etv2+Flk-1− cells suggests that Etv2 can promote hematopoiesis and vasculogenesis cell autonomously, at least in these early stages.
Etv-2 is dispensable for the differentiation of Flk1+ cells but indispensable for EC and HPC differentiation
The preceding results suggest that Etv2 marks a subset of Flk1+ cells with hemato-endothelial capacity and that Flk-1 expression can be partly independent of Etv2. To investigate the requirement of Etv2 for Flk-1 induction, Etv-2–null ESCs were differentiated on OP9 cells. Interestingly, from day 4 or 5 of differentiation, Flk-1+ cells were indistinguishable from Etv2+/− and −/− ESCs (Figure 3A). Flk-1+ cells from Etv2+/− and Etv2−/− ESCs were sorted to confirm that Etv2 RNA was lost to the background level in Etv2−/− cells by Q-PCR (Figure 4Bb). Although initial induction of Flk-1 was observed in differentiated Etv2−/− ESCs, further differentiation into HPCs and ECs was blocked. Flow cytometry revealed that Etv2−/− cells were unable to generate VE-cadherin+ or CD41+ cells (Figure 3A) and failed to down-regulate PDGFRα (Figure 3A). These results demonstrate that Etv2 is indispensable for the differentiation of VE-cadherin+ ECs or CD41+ HPCs, but is dispensable for the initial induction of Flk-1+ cells.
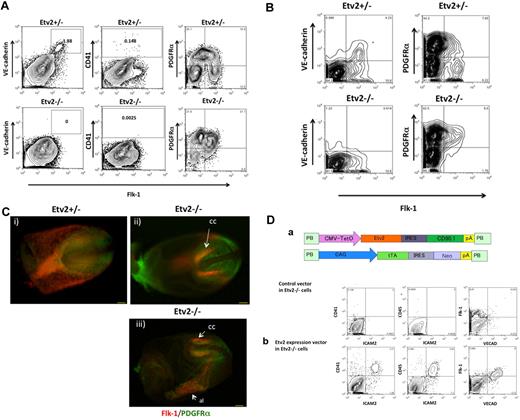
Etv2 is dispensable for the generation of Flk-1+ mesoderm but is required for the generation of HPCs/ECs. (A) Etv2+/− cells differentiated on OP9 could generate 1.88% VE-cadherin+ or 0.148% CD41+ cells (top). Etv2−/− ESCs generated Flk-1+ cells but failed to differentiate into VE-cadherin+ (0%) nor CD41+ cells (0.0025%) (bottom). Flk-1+ cells from Etv2−/− ESCs were arrested as PDGFRα+ cells. Etv2+/− Flk-1+ cells could down-regulate PDGFRα. (B) In contrast to Etv2+/− (top), E8.5 Etv2−/− cells (bottom) failed to generate Flk-1+VE-cadherin+ cells (leftpanels). Most of the Flk-1+ cells remained as PDGFRα+ in Etv2−/− embryos, failing to become Flk-1 single-positive cells (right). (C) Double immunostaining of Etv2+/− (i) and Etv−/− (ii) E7.75 mouse embryos by anti-Flk-1 (red) and anti-PDGFRα (green) Abs (iii). In the Etv2−/− embryos, Flk-1 staining in extra-embryonic mesoderm was missing, whereas Flk-1+ cells in the cardiac crescent (cc) and allantois (al) were still present. Scale bar indicates 100 μm. (D) Rescue of Etv2−/− cells by reexpression of Etv2 transgene. (a) Diagrams of Etv2 transgene constructs. PB-based tTA and Tet-inducible promoter-driven transgene vectors were transfected into Etv2-null ESCs with transgene expression monitored by CD90.1 staining. (b) HPCs (CD41+ and CD45+) and ECs (VECAD+ and Flk-1+) were generated in Etv2-rescued Etv2−/− cells (bottom), whereas none were generated in cells transfected with empty vector (top).
Etv2 is dispensable for the generation of Flk-1+ mesoderm but is required for the generation of HPCs/ECs. (A) Etv2+/− cells differentiated on OP9 could generate 1.88% VE-cadherin+ or 0.148% CD41+ cells (top). Etv2−/− ESCs generated Flk-1+ cells but failed to differentiate into VE-cadherin+ (0%) nor CD41+ cells (0.0025%) (bottom). Flk-1+ cells from Etv2−/− ESCs were arrested as PDGFRα+ cells. Etv2+/− Flk-1+ cells could down-regulate PDGFRα. (B) In contrast to Etv2+/− (top), E8.5 Etv2−/− cells (bottom) failed to generate Flk-1+VE-cadherin+ cells (leftpanels). Most of the Flk-1+ cells remained as PDGFRα+ in Etv2−/− embryos, failing to become Flk-1 single-positive cells (right). (C) Double immunostaining of Etv2+/− (i) and Etv−/− (ii) E7.75 mouse embryos by anti-Flk-1 (red) and anti-PDGFRα (green) Abs (iii). In the Etv2−/− embryos, Flk-1 staining in extra-embryonic mesoderm was missing, whereas Flk-1+ cells in the cardiac crescent (cc) and allantois (al) were still present. Scale bar indicates 100 μm. (D) Rescue of Etv2−/− cells by reexpression of Etv2 transgene. (a) Diagrams of Etv2 transgene constructs. PB-based tTA and Tet-inducible promoter-driven transgene vectors were transfected into Etv2-null ESCs with transgene expression monitored by CD90.1 staining. (b) HPCs (CD41+ and CD45+) and ECs (VECAD+ and Flk-1+) were generated in Etv2-rescued Etv2−/− cells (bottom), whereas none were generated in cells transfected with empty vector (top).
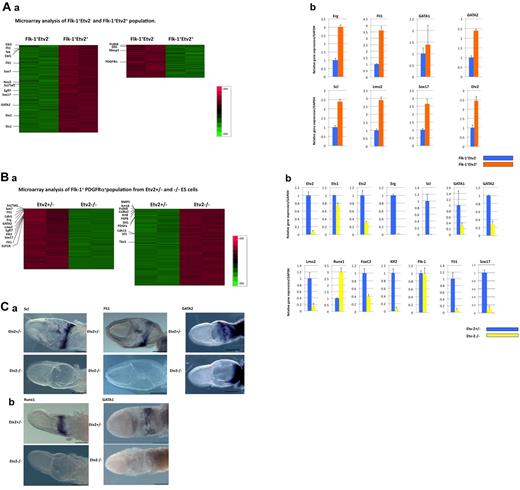
Genes regulated by Etv2. (A) Gene-expression profiling of Flk-1+Etv2+ and Flk-1+Etv2– populations generated from differentiated of Etv2-Venus+/− ESCs for 5 days on OP9. Flk-1+Etv2+ or Flk-1+Etv2− cells were analyzed by (a) microarray and (b) Q-PCR. Genes important for EC and HPC differentiation were preferentially expressed in Etv2+ Flk-1+ cells. Genes that showed significant changes between Flk-1+Etv2+ and Flk-1+Etv2− populations are listed in supplemental Table 1. (B) (a) Gene-expression analysis of Flk-1+ PDGFRα+ cells derived from Etv2+/− and −/− ESCs. After 5 days of differentiation, Flk-1+PDGFRα+cells were subjected to microarray analysis. Genes that showed significant changes between Etv2+/− and −/− populations are listed in supplemental Table 2. (b) Expression levels of key EC and HPC genes were analyzed by Q-PCR in Etv2+/−and Etv2−/− populations. Genes important for EC and HPC development were down-regulated in Etv2−/− cells. In Q-PCR experiments (Ab and Bb) PCR reactions were performed in triplicate. Data for each gene was normalized by GAPDH and are shown as the -fold change compared with Flk-1+Etv2− (Ab) or Etv2+/− (Bb) populations. (C) Expressions of Scl, Fli1, GATA2 (a), Runx, and GATA1 (b) in E7.5 Etv2+/− or Etv2−/−embryos. Scl, Fli1, and Runx: in situ hybridization; GATA1: immunostaining. Unlike expression analysis in ESC-derived Flk-1+ cells, Runx1 was undetectable in Etv2−/− embryos. GATA1 and GATA2 were down-regulated but detectable in differentiated Etv2 −/− cells but totally undetectable in Etv2−/− embryos. Scale bar indicates 300 μm.
Genes regulated by Etv2. (A) Gene-expression profiling of Flk-1+Etv2+ and Flk-1+Etv2– populations generated from differentiated of Etv2-Venus+/− ESCs for 5 days on OP9. Flk-1+Etv2+ or Flk-1+Etv2− cells were analyzed by (a) microarray and (b) Q-PCR. Genes important for EC and HPC differentiation were preferentially expressed in Etv2+ Flk-1+ cells. Genes that showed significant changes between Flk-1+Etv2+ and Flk-1+Etv2− populations are listed in supplemental Table 1. (B) (a) Gene-expression analysis of Flk-1+ PDGFRα+ cells derived from Etv2+/− and −/− ESCs. After 5 days of differentiation, Flk-1+PDGFRα+cells were subjected to microarray analysis. Genes that showed significant changes between Etv2+/− and −/− populations are listed in supplemental Table 2. (b) Expression levels of key EC and HPC genes were analyzed by Q-PCR in Etv2+/−and Etv2−/− populations. Genes important for EC and HPC development were down-regulated in Etv2−/− cells. In Q-PCR experiments (Ab and Bb) PCR reactions were performed in triplicate. Data for each gene was normalized by GAPDH and are shown as the -fold change compared with Flk-1+Etv2− (Ab) or Etv2+/− (Bb) populations. (C) Expressions of Scl, Fli1, GATA2 (a), Runx, and GATA1 (b) in E7.5 Etv2+/− or Etv2−/−embryos. Scl, Fli1, and Runx: in situ hybridization; GATA1: immunostaining. Unlike expression analysis in ESC-derived Flk-1+ cells, Runx1 was undetectable in Etv2−/− embryos. GATA1 and GATA2 were down-regulated but detectable in differentiated Etv2 −/− cells but totally undetectable in Etv2−/− embryos. Scale bar indicates 300 μm.
Etv2 is indispensable for differentiation of Flk-1+PDGFRα− vascular mesoderm from the Flk-1+PDGFRα+ primitive mesoderm
We showed previously that Flk1+PDGFRα+ double-positive primitive mesoderm (DP-PM) diverges into Flk1+ PDGFRα− vascular mesoderm (VSP), which gives rise to ECs and HPCs and Flk1−PDGFRα+ paraxial mesoderm (PSP).17,21 This ESC differentiation model can mimic the initial mesoderm differentiation and part of the primitive hematopoiesis. In this differentiation system, Etv2−/− ESCs generated Flk1+PDGFRα+ primitive mesoderm, however, they failed to further differentiate into Flk1+PDGFRα− VSP (Figure 3A). Differentiation into Flk1−PDGFRα+ PSP was unaffected. These results showed the critical role of Etv2 in the commitment of the VSP from the Flk1+PDGFRα+ primitive mesoderm.
To determine whether this observation could be recapitulated in embryos, we analyzed the expressions of PDGFRα and Flk-1 by flow cytometry (Figure 3B) and immunostaining (Figure 3C) in Etv2-null embryos. Whereas previous studies reported almost no Flk1 expression in Etv2-null embryos from the primitive streak stage to E9.5, we detected Flk-1+ cells in E8.5 embryos by flow cytometry. At E8.5, PSP and VSP commitment was nearly completed in normal embryos. However, the flow cytometric analysis demonstrated that the majority of Flk-1+ cells in Etv2-null embryos coexpressed PDGFRα and failed to generate VE-cadherin+ cells, whereas differentiation of Flk1−PDGFRα+ cells was unaffected (Figure 3B). In control embryos, the DP-PM cells were already diverged into Flk1−PDGFRα+or Flk1+PDGFRα− mesoderm (Figure 3B).
Double immunostaining for Flk-1 and PDGFRα revealed that Flk-1+ cells missing in Etv2-null embryos were Flk1+PDGFRα− cells in the extra-embryonic region where blood islands exist in normal embryos (Figure 3C). Flk-1+ cells in Etv2-null embryos were Flk1+PDGFRα+ double positive and localized in allantois, cardiac crescent,22 and a narrow overlapping lateral area between the Flk1+and PDGFRα+ domains (Figure 3C and supplemental Figure 3). We conclude that Etv2 is the key regulator of the differentiation from primitive to VSP mesoderm.
Etv2 reexpression in Etv2−/− ESCs restores hemato-endothelial differentiation
Results from Etv2−/− ESCs and embryos point to Etv2 as a master regulator of hemato-endothelial fate from the primitive mesoderm. However, it is not clear whether complete loss of HPCs and ECs is only because of Etv2 deficiency. Although zebrafish etsrp morphants were reported to be rescued by injecting etsrp mRNA, the effects of Etv2 homolog knock-down on hematopoiesis in zebrafish or Xenopus are smaller than in mice.23,24 To determine whether Etv2 can rescue the loss of HPCs and ECs in mice, we introduced an Etv2 transgene into Etv2-null ESCs (Figure 3Da). It is assumed that ESC differentiation cannot fully recapitulate the embryonic hematopoiesis, however, it has been used at least to mimic the primitive part of hematopoiesis. ESCs were differentiated in the absence of Tet to induce a transgene for which expression was monitored by CD90.1 staining. Transgene expressers were sorted by CD90.1 after 3 days of differentiation, when key hemato-endothelial factors are assumed to be required for rescue,25 and reseeded on OP9. After a total of 10 days of differentiation, cells transfected with the empty vector failed to generate VECAD+ or CD41+ cells. In contrast, the Etv2-transfected cells could generate VECAD+, CD41+, or CD45+ cells (Figure 3Db). These findings confirmed that mouse Etv2 by itself reconstitutes initial steps of vasculogenesis and hematopoiesis in the Etv2-null background.
Investigation for genes downstream of Etv2
To identify genes regulated by Etv2, we performed gene-expression profiling using Q-PCR and microarray. In the first experiment, we compared Flk-1+Etv2+ and Flk-1+Etv2− populations in differentiated Etv2-Venus+/− ESCs (Figure 4A). In the second experiment, we compared Flk-1+PDGFRα+ populations differentiated from Etv2+/− and Etv2−/− ESCs. Because most of the Flk-1+ cells from Etv2−/− cells were PDGFRα+, Flk-1+PDGFRα+, cells were sorted for comparison (Figure 4B). Genes up-regulated in Etv2-Venus+ populations but not in Etv2-Venus− include those that are important in vasculogenesis and hematopoiesis (supplemental Table 1). Many key hemato-endothelial genes that were up-regulated in Etv2-Venus+ cells in the first analysis showed profoundly reduced expression in the Etv2-null Flk-1+ cells in the second analysis (supplemental Table 2). Among the top 192 genes listed in supplemental Table 2, ∼ 50 are known to be expressed specifically in HPCs or ECs. The different expression levels of important genes were also confirmed by Q-PCR analyses (Figure 4Ab and 4Bb). Gene lists from both experiments contain an array of genes that are expressed in early HPCs, ECs, or both lineages. Interestingly, Scl1 and Fli1,2 for which null mice have profound defects in both EC and HPC differentiation, were completely deficient in Etv2-null–derived Flk-1+ cells. These 2 genes were also undetectable in Etv2-null embryos (Figure 4Ca). GATA126 and GATA2 expression levels decreased remarkably, but residual expression was detected in Etv2-null Flk-1+ cells in in vitro differentiation. Runx127 expression was almost unaffected in Etv2-null ESC-derived Flk-1+ cells. However, expressions of these genes were lost in Etv2-null E7.5 extra-embryonic mesoderm (Figure 4Cb), presumably because of the loss of the Flk-1+ cell population committed to HPCs.
Etv2 target genes are critical in vasculogenesis and hematopoiesis
Among several genes potentially regulated by Etv2, we tested Scl, Fli1, and Gata2 as critical candidates because of their expressions at sites of early embryonic hematopoiesis and functional importance in EC and HPC differentiation.1,2,28,29 We found that these 3 genes were up-regulated in Etv2-rescued Etv2−/− cells (supplemental Figure 4). Sequences matching reported Etv2-binding sites were found within ± 5 kb of the transcription start sites of Scl, Gata2, and Fli1 in both humans and mice (Figure 5A).11,30,31 Indeed, the Scl core enhancer32 has been reported to contain the binding site for Etv2.11 EMSA demonstrated the binding of Etv2 on those conserved sequences of Gata2 and Fli1, as well as Scl (Figure 5B). ChIP analysis using ECs (Figure 5C) or ESC-derived (supplemental Figure 5A) Flk-1+ cells indicated that Etv2 can bind to these sequences. Activation of each luciferase reporter for Scl, Fli1, or GATA2 was seen by Etv2 and by Ets1 and Ets2 (supplemental Figure 5B). These activations were reduced by mutated Etv2-binding sequences in each reporter except Scl, presumably because of the existence of other Ets motifs. In early embryos, Etv2 was expressed in E7.5 extra-embryonic mesoderm and in E8.5 dorsal aorta, where Scl, Fli1, and Gata2 were expressed (Figure 5D). Expressions of those genes were absent from E7.5 Etv2-null embryos, supporting the notion that Etv2 induces those genes (Figure 4Ca). Scl, Gata2, and Fli1 were reported to form a transcription factor (TF) network for HSCs/HPCs.33,34 Therefore, it is possible that Etv2 can induce these 3 genes, resulting in the induction of this TF network. To functionally test whether these genes can rescue at least the primitive part of hematopoiesis downstream of Etv2, we introduced Scl or Fli1 into Etv2-null cells because these 2 were most severely down-regulated in Etv2-null Flk-1+ cells (Figure 4Bb). Reexpression of Scl or Fli1 alone in Etv2-null cells restored CD41+CD45+ or VECAD+ cells. Transfection of empty control vector did not restore any of these cells (Figure 6A-B). Rescue experiments with GATA2 so far failed to generate CD41+ CD45+or VECAD+ cells under the same conditions (data not shown). These findings suggest that Scl and Fli1 are critical targets of Etv2 in the TF network triggered by Etv2 to generate HPCs and ECs and GATA2 is less critical.
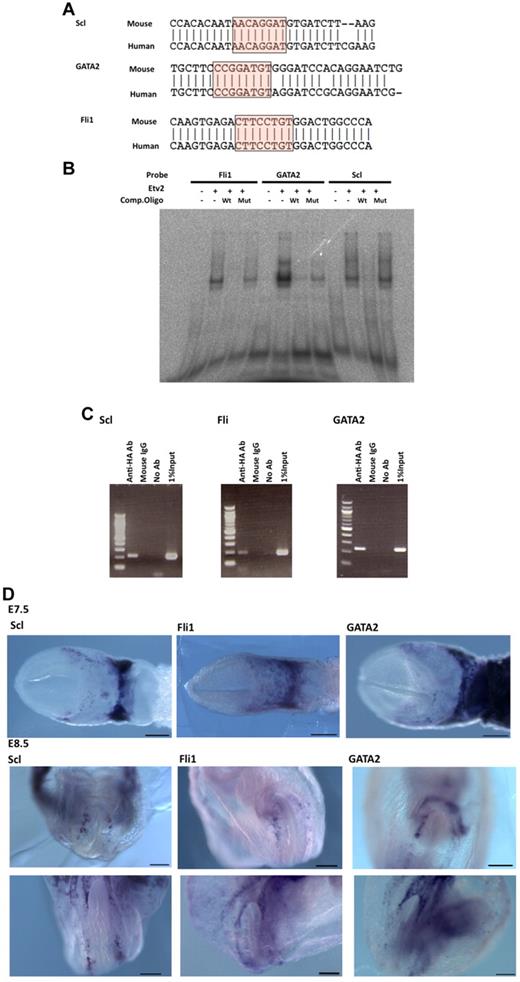
Scl, Fli1, and GATA2 are downstream targets of Etv2. (A) Alignment of candidate binding sequences containing the Ets binding motif. Putative Ets- or Fox;Ets-binding sequences from human and mouse genomes of Scl, Fli1, and GATA2 were aligned. (B) EMSA showing binding of in vitro–translated Etv2 to oligonucleotide probes containing the Ets-binding sites shown in Figure 6A for Scl, Fli1, and GATA2. Binding was competed by excess wild-type but not by Ets motif–mutated probes. (C) ChIP analysis demonstrating the binding of Etv2 to target sequences. F2 ECs were transfected with HA-tagged Etv2 expression vector and subjected to ChIP analysis. Primer set for each analysis was for Scl;Scl-1_F2/R2, Fli1;Fli1F11/R11, and GATA2;GATA2-F3/R3. (D) Expressions of Scl, GATA2, and Fli1 at E7.5 and E8.5 in mouse embryos. Expressions of Scl, GATA2, and Fli1 were examined by in situ hybridization. These 3 genes are expressed in the extra-embryonic mesoderm of E7.5 (top) and along the dorsal aorta of E8.5 embryos (middle and bottom). Scale bar indicates 200 μm.
Scl, Fli1, and GATA2 are downstream targets of Etv2. (A) Alignment of candidate binding sequences containing the Ets binding motif. Putative Ets- or Fox;Ets-binding sequences from human and mouse genomes of Scl, Fli1, and GATA2 were aligned. (B) EMSA showing binding of in vitro–translated Etv2 to oligonucleotide probes containing the Ets-binding sites shown in Figure 6A for Scl, Fli1, and GATA2. Binding was competed by excess wild-type but not by Ets motif–mutated probes. (C) ChIP analysis demonstrating the binding of Etv2 to target sequences. F2 ECs were transfected with HA-tagged Etv2 expression vector and subjected to ChIP analysis. Primer set for each analysis was for Scl;Scl-1_F2/R2, Fli1;Fli1F11/R11, and GATA2;GATA2-F3/R3. (D) Expressions of Scl, GATA2, and Fli1 at E7.5 and E8.5 in mouse embryos. Expressions of Scl, GATA2, and Fli1 were examined by in situ hybridization. These 3 genes are expressed in the extra-embryonic mesoderm of E7.5 (top) and along the dorsal aorta of E8.5 embryos (middle and bottom). Scale bar indicates 200 μm.
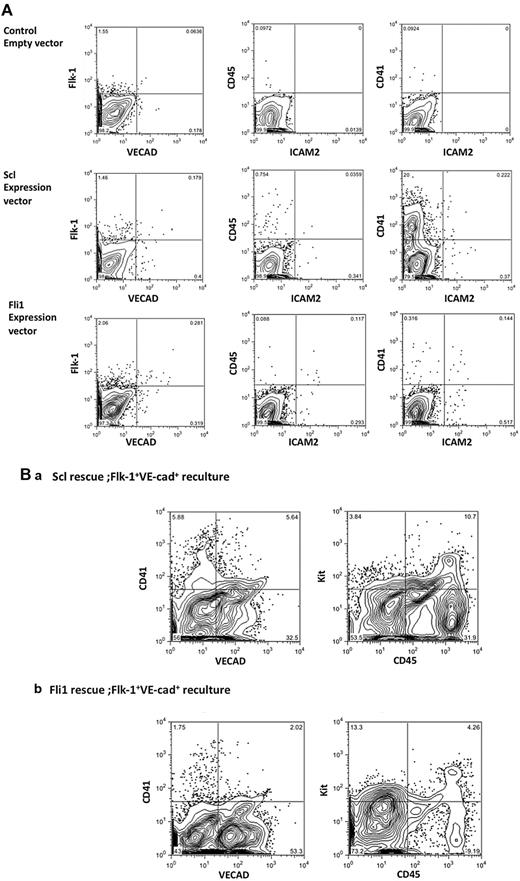
Scl or Fli rescues EC and HPC development in Etv2-null cells. (A) Expression of Scl or Fli1 alone could restore EC and HPC development in Etv2-null cells. Scl or Fli1 was introduced into Etv2-null ESCs using a Tet-inducible system. Cells differentiated on OP9 were analyzed by flow cytometry after 10 days. CD41+CD45+or VECAD+ cells developed but with lower efficiency compared with Etv2-rescued cells (except CD41+ cells in Scl rescue). (B) VECAD+ cells were sorted from Scl- or Fli1-rescued Etv2−/− differentiated cells and recultured on OP9 for an additional 8 days in the presence of HPC growth factors. This reculture generated a significant number of VECAD+ or CD45+ cells from both Scl- (a) and Fli1 (b)–rescued Etv2-null cells.
Scl or Fli rescues EC and HPC development in Etv2-null cells. (A) Expression of Scl or Fli1 alone could restore EC and HPC development in Etv2-null cells. Scl or Fli1 was introduced into Etv2-null ESCs using a Tet-inducible system. Cells differentiated on OP9 were analyzed by flow cytometry after 10 days. CD41+CD45+or VECAD+ cells developed but with lower efficiency compared with Etv2-rescued cells (except CD41+ cells in Scl rescue). (B) VECAD+ cells were sorted from Scl- or Fli1-rescued Etv2−/− differentiated cells and recultured on OP9 for an additional 8 days in the presence of HPC growth factors. This reculture generated a significant number of VECAD+ or CD45+ cells from both Scl- (a) and Fli1 (b)–rescued Etv2-null cells.
Although Scl, Gata2, and Fli1 induced by Etv2 were reported to create a core network for HSCs at E10.5-11.5 aorta-gonad-mesonephron (AGM) and E12.5 fetal liver, Etv2 could not be detected in the vessel wall in AGM and fetal liver by immunostaining or FACS (supplemental Figure 7Aa and supplemental Figure 6). A few Venus+ cells in E10.5 embryos were negative for Sca-1, a marker for HSCs (supplemental Figure 7Ab), indicating that Etv2 was not expressed in AGM HSCs.35 Despite Etv2 being undetected, Scl, Gata2, and Fli1 expressions still persisted in AGM and fetal liver cells.33 Therefore, it is possible that Etv2 is required only in the earlier stages to induce the TF network that can be maintained without Etv2 expression. This idea was also tested by analyzing expressions of these 3 genes in Etv2+VE-cadherin−, Etv2+VE-cadherin+, Etv2−VE-cadherin+, and VE-cadherin−CD41+ populations generated in ESC differentiation culture toward hematopoietic and ECs. It is assumed that Etv2+VE-cadherin−-P1, Etv2+VE-cadherin+cells-P2, Etv2−VE-cadherin+-P3, and VE-cadherin−CD41+-P4 populations represent immature mesoderm being committed to ECs, EC precursors, differentiated ECs, and differentiated HPCs, respectively (supplemental Figure 7Ba). On differentiation day 5, higher levels of Scl, Fli1, and GATA2 were seen in Etv2-Venus+ than in Etv2-Venus− cells, indicating that Etv2 can trigger the network of these 3 genes. At day 6, as cells differentiated into mature ECs or HPCs, a significant number of VE-cadherin+ and CD41+ cells appeared in culture (supplemental Figure 7Ba). Q-PCR revealed that Scl, Fli1, and GATA2 were expressed at similar levels in Etv2+VE-cadherin+ and Etv2−VE-cadherin+ cells and that Fli1 was down-regulated in differentiated HPCs represented by VE-cadherin−CD41+cells. Erg, reported to be important in hemangioblast and HSC development,36 was regulated in a similar manner to Fli1 (supplemental Figure 7Bb). This expression profile indicates that the Scl-GATA2-Fli1 network is induced by Etv2, that the established network can be maintained with lower expression of Etv2, and that Fli1 is down-regulated during HSC/HPC generation as cells begin to lose their EC phenotype. To further support the transient requirement of Etv2, part of the VE-cadherin+ or CD41+ cells from rescued Etv2-null cells could exist in CD90.1low or CD90.1− fraction after transgene suppression, indicating that cells do not require continuous higher expression of Etv2 to generate HPCs or ECs (supplemental Figure 8).
Etv2 is an immediate responding gene in VEGF-treated Flk-1+ primitive mesoderm
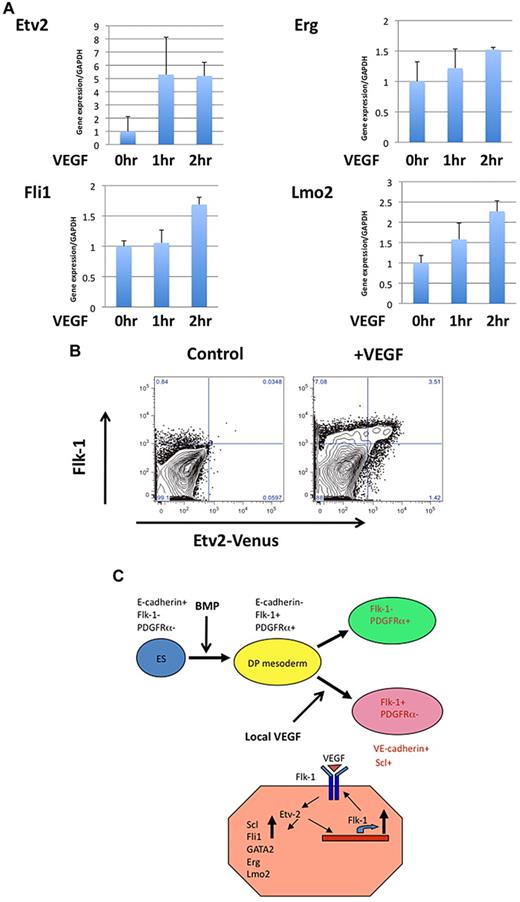
Based on the finding that Etv2 was dispensable for initial Flk-1+ induction but was required for the further differentiation of Flk-1+ cells into HPCs/ECs, we reconsidered the way that Etv2 is induced. Wnt, BMP, or Notch signaling pathways were shown to up-regulate Etv2. Although these pathways also contribute to HSC development, we reasoned that Etv2 induction is correlated with Flk-1 expression. It was reported that VEGF is expressed in the E7.0-E8.5 extra-embryonic endoderm close to the Flk1+ extra-embryonic mesoderm, where Etv2+ cells were first detected.37 By stimulating ESC-derived Flk-1+ mesoderm with VEGF, we found that VEGF potently (∼ 5-fold by Q-PCR) induced Etv2 in these cells (Figure 7A). Because this increase occurred within 1 hour, we considered this to be the direct effect over Etv2 transcription rather than selective proliferation of Etv2+ cells. Fli1 and Lmo2, a cofactor of Scl, were up-regulated within 2 hours (Figure 7A). VEGF treatment for 24 hours also increased the number of Etv2+ cells among the Flk1+ population (Figure 7B). These effects of VEGF will generate a positive feedback of VSP specification because Etv2 can in turn up-regulate Flk-1 expression.
Etv2 is an immediate-response gene in VEGF-treated Flk-1+ mesoderm. (A) ESC-derived Flk-1+ cells maintained in serum-free medium were treated with VEGF (20 ng/mL) for the indicated periods of time. Gene expressions were quantified by Q-PCR. (B) VEGF(20 ng/mL) treatment for 24 hours significantly increased the Etv2+ cells in differentiating ESCs. In addition to an increase in Flk-1+ cell numbers, the number of Etv2+ cells among Flk-1+ cells was also increased. (C) Mesoderm differentiation and role of Etv2 in promoting VSP formation. Primitive mesoderm generated as the Flk-1+PDGFRα+ DP-PM population diverges into Flk-1+ PDGFRα− VSP and Flk-1− PDGFRα+ PSP. Etv2 is required for the progression of DP-PM into VSP. VEGF-Flk-1 signaling promotes VSP induction by up-regulating Etv2 expression. Etv2 triggers VSP induction and subsequent EC and HPC generation by inducing a series of hemato-endothelial genes, Scl, GATA2, Fli1, Lmo2, and Erg.
Etv2 is an immediate-response gene in VEGF-treated Flk-1+ mesoderm. (A) ESC-derived Flk-1+ cells maintained in serum-free medium were treated with VEGF (20 ng/mL) for the indicated periods of time. Gene expressions were quantified by Q-PCR. (B) VEGF(20 ng/mL) treatment for 24 hours significantly increased the Etv2+ cells in differentiating ESCs. In addition to an increase in Flk-1+ cell numbers, the number of Etv2+ cells among Flk-1+ cells was also increased. (C) Mesoderm differentiation and role of Etv2 in promoting VSP formation. Primitive mesoderm generated as the Flk-1+PDGFRα+ DP-PM population diverges into Flk-1+ PDGFRα− VSP and Flk-1− PDGFRα+ PSP. Etv2 is required for the progression of DP-PM into VSP. VEGF-Flk-1 signaling promotes VSP induction by up-regulating Etv2 expression. Etv2 triggers VSP induction and subsequent EC and HPC generation by inducing a series of hemato-endothelial genes, Scl, GATA2, Fli1, Lmo2, and Erg.
Discussion
Flk-1+ cells from ESCs or early embryos represent a broader spectrum of mesoderm precursors than those giving rise only to ECs or to HPCs.38-41 These findings suggest that Flk-1 is not a specific marker for EC/HPC precursors, nor is it absolutely required for their development. Etv2/ER71 completely blocks the differentiation of HPCs/ECs in mice, which was attributed to the dependence of Flk-1 transcription on Etv2.10 However, this interpretation does not explain the complete loss of ECs and HPCs in Etv2-null mice, which does not happen in Flk-1–null cells. We showed that Etv2 is dispensable for the initial Flk-1 induction in Flk-1+PDGFRα+ (DP-PM), but critical for the generation of Flk-1+PDGFRα− (VSP) mesoderm. VSP failed to differentiate from DP-PM in Etv2-null ESCs and embryos. In contrast, differentiation of Flk-1−PDGFRα+ (PSP) mesoderm seemed normal in the absence of Etv2. Although some mesenchymal or muscle-specific genes were up-regulated in Etv2−/− cells, no obvious expansion of the PDGFRα+ domain was seen in Etv2−/− embryos. Therefore, it is possible that up-regulation of mesenchymal or muscle genes in Etv2 −/− cells may be partly due to the loss of VSP population. Nonetheless, it is also possible that mesenchymal or muscle genes are derepressed in the absence of Etv2, because Etv2 can work as a transcriptional repressor by interacting with TGA.42 Flk-1+PDGFRα+ mesoderm, which normally exists only transiently, was retained longer in Etv2-null–differentiated ESCs and embryos. We also found that VEGF is a potent and rapid inducer of Etv2 in Flk-1+cells. Etv2+ cells appeared exclusively in the posterior area of the Flk1+ cell cluster around E7.0 (Figure 1A), suggesting that Flk-1 expression might underlie Etv2 induction. VEGF expression was reported in extra-embryonic endoderm and over the cardiac crescents adjacent to the Flk-1+ cells.37 We propose that VEGF induces Etv2 expression in Flk-1+ mesoderm and that Etv2 in turn up-regulates Flk-1 to stabilize the VSP fate (Figure 7C).
Comparison of genes expressed in Flk1+ cells derived from Etv2+/− and Etv2−/− ESCs revealed that genes critical in HPC/EC differentiation were up-regulated by Etv2, which is consistent with results in etsrp-overexpression experiments in zebrafish.14,15 Among them, Scl, Fli1, and Gata2 were analyzed because of their functional importance in forming a TF network to direct EC/HSC differentiation.1,2,28,29 We showed that Scl, Fli1, and GATA2 can be up-regulated by Etv2 and that Scl or Fli1 alone could restore HPCs and ECs in Etv2-null cells. Whereas Fli1 rescue experiments in zebrafish failed to achieve Runx1 expression,43 in Etv2-null ESCs Fli1 could rescue HPCs to generate Runx1-dependent CD45+ cells,44 suggesting that Fli1 can be as functional as Scl. We did not observe CD41+ or CD45+ cells in GATA2-rescued Etv2-null cells. Some VECAD+ but ICAM2− cells appeared transiently in GATA2-rescued Etv2-null cells; however, these cells failed to develop CD41+ or CD45+ cells. GATA2 also induced lower levels of Scl or Fli1 expression in Etv2-null cells, indicating that there might be partial rescue (data not shown). Our study also suggested a transient requirement for Etv2 to generate ECs/HPCs. Several endothelial genes considered to be downstream targets of Etv2 (supplemental Table 2), including VE-cadherin, maintained their expressions in ECs even after Etv2 down-regulation. Likewise, in experiments to rescue Etv2-null cells, some VECAD+ or CD41+ cells exist as a CD90.1− or CD90.1low population, representing cells of lower Etv2 expression (supplemental Figure 8). Because ESC differentiation cannot fully recapitulate embryonic hematopoiesis, these insights may not be directly applicable to the in vivo process. However, the transient expression of Etv2 also observed in developing embryos supports this view. We propose that, as a master regulator, Etv2 induces several HPC/EC genes, creating a cell-intrinsic status of HPC/EC development that can be maintained even after down-regulation of Etv2.
Consistent with its transient requirement, Etv2 expression was detectable only in the beginning phase of vasculogenesis/hematopoiesis for a restricted period of embryogenesis. Etv2 was obviously expressed in the E7.5 yolk sac and the E8.5 aorta and posterior part of the embryo (corresponding to splanchnopleure), but was barely detectable in E10.5 AGM and E12.5 fetal liver (supplemental Figure 7A and supplemental Figure 6). It is possible that other Ets family members may work in AGM or fetal liver as substitutes.45 However, no other Ets genes, including Fli1, Ets1, and Ets2, have been reported to cause such severe hematopoietic defects as Etv2, indicating the unique role of Etv2.46-48 The lack of Etv2 expression in AGM and fetal liver might indicate the role of Etv2 mostly in the yolk sac hematopoiesis but not in later stages. Because Etv2 seems to be required transiently during HPC/EC development and to promote vasculogenesis and hematopoiesis in Flk-1+ cells from E7.5 extra-embryonic mesoderm and E8.5 embryo proper parts, it is also possible that transiently expressed Etv2 initiates hemato-endothelial TFs in precursors present in yolk sac,49 splanchnopleure, or dorsal aorta that give rise to AGM and fetal liver HSCs. Defining where Etv2 specifies HSCs needs further investigation.
AGM ECs have been shown to generate HPCs as hemogenic ECs, whereas some HPCs may be generated directly from common EC/HPC precursors called “hemangioblasts.” At present, no molecular marker can clearly distinguish between hemogenic ECs and hemangioblasts. Etv2 expression elevated in early mesoderm committed to ECs/HPCs but down-regulated in differentiated cells including AGM ECs suggests that it can mark hemangioblasts. However, in zebrafish, etsrp (the Etv2 homolog) deficiency affects EC development more than that of HPCs, indicating that Etv2/Etsrp expression may represent endothelial precursors rather than common EC/HPC precursors.50
Regarding the role of Etv2 in hematopoiesis, we observed a discrepancy in the expression of GATA1 and Runx1 between Etv2−/− embryos and in vitro–differentiated Flk-1+ cells. GATA1 and Runx1 expressions were undetectable in E7.5 extra-embryonic mesoderm of Etv2−/− embryos (Figure 4Cb), whereas Etv2-null ESCs differentiated on OP9 retained detectable levels of both genes (Figure 4Bb). Impaired Runx1 expression was also reported in the zebrafish etsrp morphant, but not in the Xenopus Etv2 knock-down.23 Therefore, GATA1 and Runx1 may be influenced by Etv2 but still be induced without Etv2 because they may not be direct targets of Etv2.14,15,23,24 Runx1 and GATA1 can promote hematopoietic development from the Etv2-induced VSP. Indeed, GATA1 is known to displace GATA2 from the same DNA-binding site to disrupt the Scl/Fli1/Gata2 network and drive the VSP to the primitive erythrocyte fate rather than the EC fate.51 In this model, GATA1 and Runx1 promote hematopoiesis from VSP by antagonizing the EC differentiation by Scl/Fli1/Gata2 network created by Etv2.
In conclusion, Etv2 is required for the induction of Flk-1+ PDGFRα− VSP but is dispensable for the initial establishment of DP mesoderm. Without Etv2, mesoderm cells are trapped as Flk-1+ PDGFRα+ DP-PM, failing to differentiate into Flk-1+PDGFRα− VSP. VEGF-Flk-1 induces Etv2, creating a positive feedback loop for vascular mesoderm specification (Figure 7C). Flk-1 is not the critical primary target of Etv2; instead, more hemato-endothelial specific genes including Scl and Fli1 are important targets. Finally, transient Etv2 expression can create the TF network to direct the generation of ECs and HPCs, which persists even after Etv2 is down-regulated (Figure 7C). These insights highlight the possibility that mesoderm derivatives such as ECs, HPCs, and cardiomyocytes can be induced in a more controlled manner from ESC or induced pluripotent stem cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs K. Uno, T. Kasukawa, J. Nishio, and C. Imai (Functional Genomics, RIKEN CDB) for microarray analysis; Drs M. Ikeya, Y. Sasai, and H. Niwa (RIKEN CDB) for providing plasmids; Dr M. Hirashima (Kobe University) for helpful comments; and Hiroshi Kiyonari and Kazuki Nakao (RIKEN CDB LARGE) for mice generation.
This work was supported by grants from the PRESTO (Japan Science and Technology Agency), Leading Project for Realization of Regenerative Medicine and Ministry of Education, Culture, Sports, Science and Technology (20590295).
Authorship
Contribution: H.K. and S.-I.N. conceived and designed the experiments and wrote the manuscript and M.H., R.N., N.I., M.L.J., Y.T., S.N., and H.T. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroshi Kataoka or Shin-Ichi Nishikawa, Laboratory for Stem Cell Biology, RIKEN Center for Developmental Biology, 2-2-3, Minatojima-Minamimachi, Chuo-ku, Kobe 650-0047, Japan; e-mail: kataokah@cdb.riken.jp or nishikawa@cdb.riken.jp.