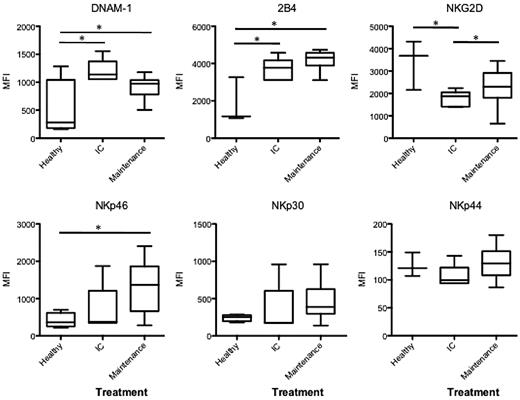
We are encouraged by Carter et al's1 findings in support of our initial work of natural killer (NK) cell dysfunction on exposure to dexamethasone in patients with multiple myeloma (MM).2 Our original study showed that dexamethasone abrogated the NK cell– stimulatory arm of lenalidomide by exhausting CD4 T cell– derived IL-2 production and inhibition of NK cell–activating receptor (NKG2D and NKp46) expression. These receptors are required for NK-cell killing of MM cells.3 Carter at al have now shown a similar effect on NK cell–activating receptors in vivo. In their study, patients with MM who received 160 mg (low dose) or 480 mg (high dose) dexamethasone per cycle, in addition to the standard lenalidomide dose (25 mg/d), had reduced expression of NK cell–activating receptors (NKG2D, NKp46, and NKp30) from baseline through to cycle 10 of therapy. In contrast to this, we have recent data on patients with newly diagnosed MM who received induction therapy with standard lenalidomide with very low dexamethasone dose (60 mg/cycle). In this dataset, NK cell– activating receptor (NKG2D, NKp46, NKp30, NKp44, and 2B4) expression is not decreased by successive therapy cycles (Figure 1). As reported previously, we show decreased NKG2D expression on NK cells4 in MM patients compared with healthy donors; however, this was restored when dexamethasone was ceased at the onset of maintenance lenalidomide therapy.
NK cell–activating receptors in newly diagnosed patients treated with lenalidomide and ultra low dose dexamethasone. NK cell–activating receptors (DNAM-1, 2B4, NKG2D, NKp46, NKp30 an NKp44) were analyzed on CD16+CD56+ NK cells from 5 newly diagnosed patients on induction therapy (IC) receiving 25 mg/d lenalidomide and 60 mg/cycle of dexamethasone, and 13 newly diagnosed patients on maintenance therapy with lenalidomide only. Data are presented as mean fluorescence intensity (MFI), a significant difference in MFI between groups is represented by * (P = < .05).
NK cell–activating receptors in newly diagnosed patients treated with lenalidomide and ultra low dose dexamethasone. NK cell–activating receptors (DNAM-1, 2B4, NKG2D, NKp46, NKp30 an NKp44) were analyzed on CD16+CD56+ NK cells from 5 newly diagnosed patients on induction therapy (IC) receiving 25 mg/d lenalidomide and 60 mg/cycle of dexamethasone, and 13 newly diagnosed patients on maintenance therapy with lenalidomide only. Data are presented as mean fluorescence intensity (MFI), a significant difference in MFI between groups is represented by * (P = < .05).
It seems that the critical variable in these 2 analyses is the dose of dexamethasone administered. Patients who received either low- or high-dose dexamethasone (160 or 480 mg/cycle) displayed down-regulated NK cell–activating receptors in vivo. In contrast, we reported that patients who received very low dexamethasone had no therapy-induced change in NK cell–activating receptors with the exception of DNAM-1. Collectively, these observations indicate that the dose of dexamethasone delivered impacts in variable ways on expression of NK cell–activating receptors in vivo, and therefore may limit the immunologic control of MM. The increasing realization of the negative impact of dexamethasone on IMiD therapy highlights the important role of translational studies as an adjunct to clinical trial observation.5
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Neeson, Cancer Immunology Program, Peter MacCallum Cancer Centre, East Melbourne, Victoria 3002, Australia; e-mail: paul.neeson@petermac.org.