Abstract
Abstract 793 FN2
FN2
Myelofibrosis (MF) is a myeloproliferative neoplasm associated with splenomegaly, debilitating symptoms, cytopenias and progressive bone marrow fibrosis that leads to early death. Patients (pts) with high-risk MF according to International Prognosis Scoring System (IPSS) have particularly poor outcome with a median survival of 2 years (yrs). No approved or effective therapy exists. Ruxolitinib is a JAK1 and JAK2 inhibitor with established clinical benefit in the treatment of pts with MF by reducing spleen size and improving quality of life.
The objective of this analysis was to compare assorted outcomes of MF pts receiving ruxolitinib to those of a matched historical control group.
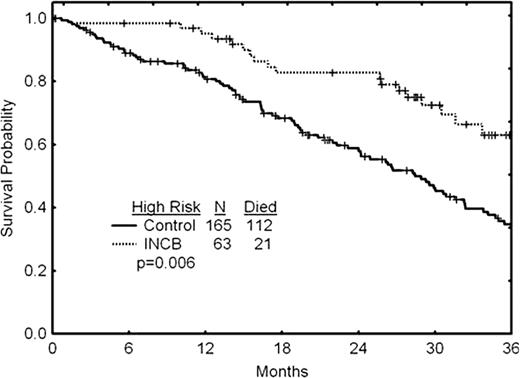
Overall survival (OS) of 107 pts enrolled in a Phase I/II trial (INCB18424-251; NCT00509899) and followed at the MD Anderson Cancer Center (MDACC) was compared to that of 310 pts with MF identified in 3 large databases (MDACC, U. of Pavia and Hospital Niguarda cà Granda, Milano) with characteristics that would have allowed them to enroll in INCB18424-251. Thus, the pt features between the 2 groups were matched based on enrollment criteria. Among 107 ruxolitinib treated pts, 63 had high risk, 34 intermediate (int)-2 and 10 int-1 risk according to IPSS. In the control group (n=310), 165 pts had high and 145 pts int-2 risk; most pts were treated with conventional or investigational therapies during follow-up.
Ruxolitinib-treated pts had a median age of 66 yrs, hemoglobin (Hb) of 10.2 g/dL, WBC of 19×10^9/L, platelets of 277×10^9/L, and palpable spleen of 19 cm. Control pts had a median age of 70 yrs, Hb of 9.7 g/dL, WBC of 12×10^9/L, platelets of 265×10^9/L, and palpable spleen of 6 cm. Baseline characteristics that differed between 2 groups included: significantly more int-2 vs high-risk pts (according to both IPSS and dynamic IPSS [DIPSS]), older age and lower Hb in the controls, as contrasted to higher WBC and larger spleen in those on ruxolitinib. There were no differences between the groups with regard to male:female ratio, platelet count, and cytogenetic characteristics. With regard to OS comparison between the 2 groups, a significant difference was seen in favor of ruxolitinib (p=0.022). Indeed, 33 of 107 pts (30.8%) in the ruxolitinib group vs. 189 of 310 (60.9%) in the control group died, after a median follow-up of 32 and 22 months, respectively. The difference in OS was highly significant in the high-risk pt subgroup (p=0.006), in that 21/63 (33.3%) vs. 112/165 (67.9%) died in the ruxolitinib and control groups, respectively. In the univariate analysis, significant factors associated with longer OS were int-2 (vs. high) risk (per IPSS/ DIPSS), platelets >400×10^9/L, and age <65 years, but not gender, abnormal cytogenetics, high WBC (>25×10^9/L), anemia (Hb <10g/dL), or spleen size. In the multivariate analysis, using age and blood cell counts as continuous variables, independent significant factors for better survival were IPSS int-2 risk, younger age, higher platelets, and treatment with ruxolitinib (p=0.02; HR=0.63).
We have identified a historical control group of pts with clinical characteristics that would have allowed them to participate in the Phase I/II study of ruxolitinib in MF. We compared their survival to 107 pts who participated in that trial, and were followed at the MDACC. The survival of pts with high-risk MF that were treated with ruxolitinib was found to be significantly longer than that of the matched control group. Further, ruxolitinib therapy was identified as an independent factor influencing better survival in the multivariate analysis. Our data suggest the potential of ruxolitinib to change the natural progression of MF pts with advanced disease.
Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract