Abstract
Abstract 4041
Hematopoietic stem cell transplantation is a commonly used treatment for various hematological malignancies. However, a life-threatening complication following this therapy is the development of Graft versus Host Disease (GvHD), during which transplanted donor immune cells attack the host and lead to severe inflammatory responses and tissue damage. Given the key role of regulatory T cells (Tregs) in immune homeostasis and peripheral tolerance, the adoptive transfer of these cells may represent a promising therapeutic opportunity for the treatment of GvHD. This approach has been proven successful to enhance graft acceptance and prevent experimental GvHD in several animal models. However, it becomes increasingly evident that antigen-specific Tregs are more efficient than polyclonal Treg populations. The antigen-specificity enables the cells to exert their suppressive effect locally at the appropriate sites of inflammation. Furthermore, the application of antigen-specific Tregs might lower the risk of unfavourable systemic immunosuppression. Nevertheless, one of the main obstacles for their clinical use is the isolation and expansion of therapeutically relevant numbers of antigen-specific Tregs.
In light of these arguments, bispecific antibodies (bsAb) could provide a promising tool for a target-dependent tissue specific redirection of polyclonal Tregs. BsAb redirect T cells to target cells by cross-linking their activating CD3 receptor and any chosen antigen on the surface of the target cell. Several studies have proven that CD8+ and CD4+ effector T cells can be successfully activated by bsAb both in vitro and in vivo. However, so far nobody has ever investigated whether Tregs can be redirected with bsAb.
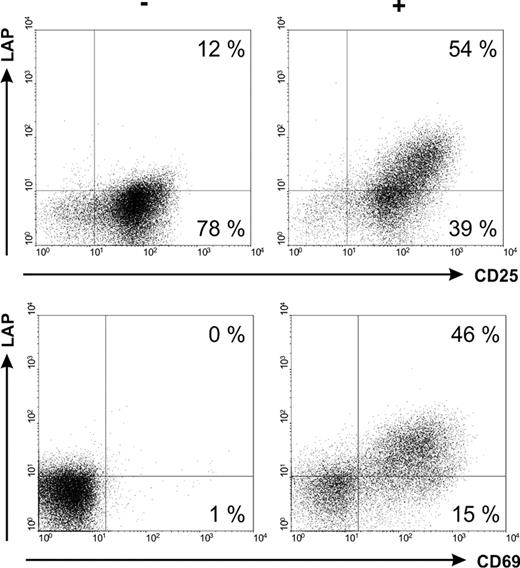
Expression of different activation associated markers on Tregs incubated without (−) or with a bsAb (+) and the respective target antigen.
Expression of different activation associated markers on Tregs incubated without (−) or with a bsAb (+) and the respective target antigen.
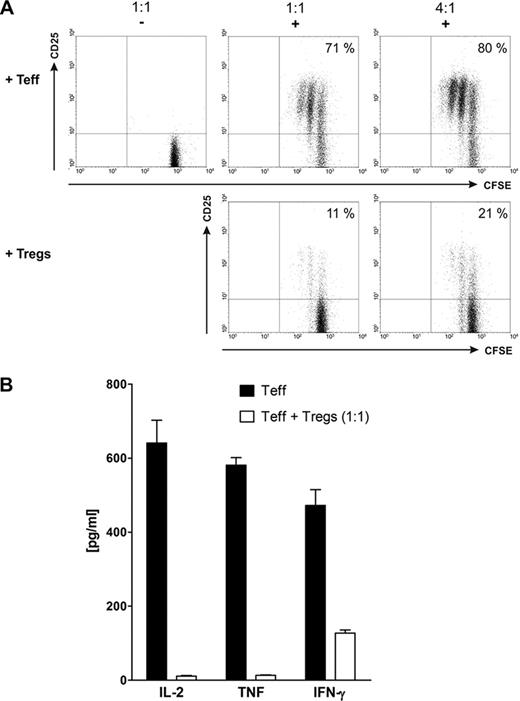
A) CFSE-labeled effector T cells (Teff) were cultured together with either unlabeled autologous effector T cells or Tregs at effector:suppressor ratios of 1:1 or 4:1 in the presence (+) or absence (−) of a bsAb and the respective target antigen. B) Cytokine secretion of bsAb-activated Teff in the presence (white bars) or absence (black bars) of autologous bsAb-activated Tregs.
A) CFSE-labeled effector T cells (Teff) were cultured together with either unlabeled autologous effector T cells or Tregs at effector:suppressor ratios of 1:1 or 4:1 in the presence (+) or absence (−) of a bsAb and the respective target antigen. B) Cytokine secretion of bsAb-activated Teff in the presence (white bars) or absence (black bars) of autologous bsAb-activated Tregs.
Taken together, we give evidence for the first time that bsAb can redirect Tregs against a surface antigen independently of their T cell receptor specificity. In view of these results, an antigen- and/or site-specific retargeting of Tregs using bsAb may open novel therapeutic approaches for a long-term establishment of tolerance against allogenic transplants and therefore would offer a new treatment option for severe GvHD after allogenic stem cell transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.