Abstract
Abstract 2947
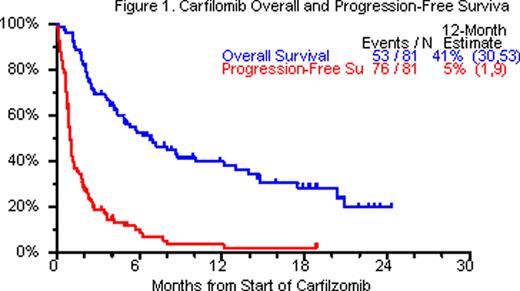
CFZ is an epoxomicin derivative with ability to irreversibly inhibit proteosomes. It has been shown in preclinical and early clinical studies to have activity in newly diagnosed as well as RRMM. We have previously reported on the UARK compassionate use phase II study of CFZ which allowed for the addition of other anti-MM drugs in RRMM. We are now presenting data with 7 additional patients and longer follow-up, with data cut-off on August 9 2011. Methods: All patients with relapsed or resistant refractory multiple myeloma were eligible for the trial. First cycle CFZ was given at 20mg/m2 IV day 1, 2, then 27mg/m2 IV days 8, 9, 15, 16 every 28 days; 4mg of dexamethasone (DEX) was given with each CFZ dose. In the absence of at least PR, CFZ dose was escalated to 36 mg/m2 IV and DEX increased to 20mg. Additional anti-myeloma drugs were added Cycle 2 onwards in absence of PR. 16-day continuous infusion cisplatin (3–5 mg/m2/d) and doxorubicin (1.5–3 mg/m2/d) were commonly added from Cycle 2 onwards, along with other novel agents. Cox regression modeling was employed for overall survival (OS) and progression free survival (PFS). Results: 81 patients with RRMM were enrolled. Baseline characteristics included age >=65yr in 32%, ISS stage >=II was seen in 72% of patients, cytogenetic abnormalities (CA) in 68%, and GEP-defined high risk in 53% of patients. 77 patients had prior autologous stem cell transplant. 63 patients (78%) had at least 2 transplants. All 81 patients had received regimens containing bortezomib, thalidomide, lenalidomide, melphalan or steroids. At least 1 cycle of treatment was administered to all 81 patients enrolled, 69% of patients received >1 cycle of treatment and only 19% received >5 cycles. 71 patients (88%) discontinued therapy primarily due to progression, death or toxicity. 5% patients achieved nCR/CR/sCR, additional 19% patients had stable disease. OS benefit was observed in patients receiving >= cycle 3 (HR= 0.40, p=0.006) on univariate analysis and multivariate analysis (HR=0.09, p<0.001) with adjustment for GEP-defined risk status. Most common toxicities, counting all toxicities (>=grade 3) were thrombocytopenia (84%), anemia (77%), leukopenia (73%), hypophosphatemia (58%), hypokalemia (27%) and fatigue (25%). Grade 1–2 peripheral neuropathy (PN) was present at baseline in 53%, >grade 3 PN was observed in 7% (6/81) after cycle 1, whereas >grade 3 PN was observed in 8% (1/12) >5 cycles.OS and PFS at 12 months were 41% and 5%, respectively (Figure 1). Conclusions: The data presented herein, confirm and extend the previously reported results on CFZ demonstrating anti-myeloma activity and clinical benefit, alone and in combination with other agents, in our heavily pre-treated RRMM population. Surprisingly, worsening or new PN was not observed in majority of patients. Utilizing the novel proteasome inhibitor CFZ in combination with other anti-MM agents on a compassionate basis afforded us the opportunity to make observations regarding potential clinical synergy of particular combinations. In particular, combination of CFZ-DEX with lenalidomide and vorinostat has shown promise in a subset of RRMM patients.
Barlogie:Celgene: Consultancy, Honoraria, Research Funding; IMF: Consultancy, Honoraria; MMRF: Consultancy; Millennium: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy; Novartis: Research Funding; NCI: Research Funding; Johnson & Johnson: Research Funding; Centocor: Research Funding; Onyx: Research Funding; Icon: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.