Abstract
Clinical evidence has shown that FLT3 internal tandem duplication (ITD) mutation confers poor prognosis in acute myeloid leukemia. Loss of the FLT3 wild-type (WT) allele is associated with even worse prognosis. We have previously reported that heterozygous FLT3wt/ITD “knockin” mice develop a slowly fatal myeloproliferative neoplasm (MPN). To study the roles of the WT FLT3 and ITD alleles in the development of MPNs, we generated FLT3/ITD homozygous (FLT3ITD/ITD) and hemizygous (FLT3−/ITD) mice. FLT3−/ITD mice, with the loss of WT allele, display a more severe MPN, as evidenced by even larger spleen, higher white blood cell counts, and shorter survival, compared with FLT3wt/ITD mice. Reintroduction of the WT FLT3 allele into FLT3−/ITD BM slowed the progression of MPN in recipient mice. FLT3ITD/ITD mice had an even severe MPN compared with the FLT3−/ITD and FLT3wt/ITD mice. Spontaneous leukemia developed in a small fraction of the FLT3ITD/ITD mice but was never observed in the FLT3−/ITD and FLT3wt/ITD mice. Our results suggest that loss of the WT allele contributes to the development of a more severe phenotype. Thus, the WT FLT3 allele seemingly functions as a tumor suppressor, attenuating the function of the FLT3/ITD allele in leukemia harboring FLT3/ITD mutations.
Introduction
Activating mutations of FLT3, either in the form of internal tandem duplication (ITD) mutations in the juxtamembrane domain or point mutations in the kinase domain, are one of the most frequent mutations in acute myeloid leukemia (AML). AML patients with FLT3/ITD mutations have an increased incidence of leukocytosis, decreased disease-free survival, and decreased overall survival.1-4 Loss of the wild-type (WT) FLT3 allele, usually with biallelic FLT3/ITD mutation, occurs in 10%-50% of FLT3/ITD leukemia cases. These patients have a significantly higher peripheral WBC count and a statistically significantly inferior clinical outcome compared with those FLT3/ITD AML patients with the WT FLT3 allele still present.5-8 In addition, FLT3/ITD patients with a high mutant-to-WT ratio have a significantly worse outcome than FLT3/ITD patients with a lower ratio.7
One possible reason for the loss of WT allele is loss of heterozygosity (LOH). Copy neutral (CN) LOH is a common event in both hematologic and solid tumors.9 It may result in inactivation of tumor-suppressor genes and/or activation of oncogenes, which in turn lead to uncontrolled cell proliferation and metastasis. In hematopoietic malignancies, acquired CN-LOH has been associated with the loss of normal allele and concomitant duplication of oncogenic mutations.9-14 FLT3 is one of the genes associated with acquired CN-LOH.8 By use of a genome-wide single nucleotide polymorphism-based array assay, homozygous mutations of FLT3 were detected in AML patients with FLT3/ITD mutations, indicating that LOH is a frequent event in these patients.15 Acquired CN-LOH, and the resultant homozygous mutation of FLT3/ITD, is also a common event associated with relapse of AML in patients who were originally heterozygous at diagnosis.16 These findings provide evidence that FLT3/ITD mutations are a manifestation of cellular events leading to genetic instability. It also suggests that loss of the WT FLT3 allele might contribute to relapse and poor prognosis in these patients.
FLT3/ITD mutations confer constitutive autophosphorylation of the FLT3 receptor and phosphorylation of its downstream targets.17 Accumulating data suggest that, as part of its pathophysiology, FLT3/ITD mutations might also activate unique downstream signaling pathways that are different from those activated by the physiologically normal WT FLT3. Microarray assays have revealed marked differences in gene expression profiles between mouse hematopoietic cell lines transduced with WT FLT3 compared with FLT3/ITD mutants.18 In contrast to signaling by the ligand-induced WT FLT3 activation, FLT3/ITD mutations are reported to repress key myeloid transcription factors and cause a block of myeloid differentiation.18-20 Hematopoietic cells transduced with FLT3/ITD also show high levels of STAT5 activation, whereas FL stimulation of WT FLT3 only results in transient and weak STAT5 phosphorylation.21 Investigating the roles of the WT allele in FLT3/ITD-associated leukemia will help to understand its prognostic implications in FLT3/ITD-related leukemia and better our understanding of leukemogenesis caused by FLT3/ITD mutations.
Recently, we and others have generated FLT3/ITD knockin mouse models.22,23 Mice heterozygous for FLT3/ITD mutation demonstrated signs of myeloproliferative neoplasms (MPNs). To study the roles WT FLT3 play in the development of leukemia associated with FLT3/ITD mutations, we crossed FLT3wt/ITD mice with themselves or with FLT3 “knockout” (FLT3−/−) mice to obtain hemizygous (FLT3−/ITD) or homozygous (FLT3ITD/ITD) FLT3/ITD mice. Investigating phenotypic differences among them reveals the impact of WT FLT3 on the development of MPN resulting from FLT3/ITD mutations, and by extension, the effect on acute leukemia.
Methods
Mice
FLT3/ITD knockin mice were generated as previously reported.23 Mice were bred with CMV-Cre transgenic mice (The Jackson Laboratory) to excise the PGK-Neo cassette. FLT3−/− mice were a kind gift from Dr Ihor Lemischka (Princeton, NJ).24 FLT3wt/ITD mice were crossed with each other to obtain the FLT3ITD/ITD mice. FLT3−/ITD mice were generated by crossing FLT3wt/ITD mice with FLT3−/− mice. All mice were maintained on a C57BL/6 background and housed in microisolator cages in a pathogen-free animal facility. All procedures were approved by the Johns Hopkins Animal Care and Use Committee.
Flow cytometric analysis and cell sorting
Flow cytometric analysis was performed as described previously23 using a BD FACSCalibur or LSRII. Cell sorting was performed on a FACSAria (BD Biosciences). Antibodies used for the staining of cell surface markers are: anti–mouse CD11b (Mac-1, M1/70), Gr1 (RB6-8C5), Ter119 (Ter119), CD45R/B220 (RA3-6B2), CD43 (S7), CD24 (M1/69), and CD19 (MB19-1, eBioscience); CD93 (AA4.1), Sca-1 (D7), CD117 (c-KIT, 2B8), CD135 (A2F10.1), and CD34 (RAM34, eBioscience); FcγRII/III (HM48-1), pSTAT5 (pY694, clone 47), and myelperoxidase (Hycult Biotech); and biotin mouse lineage panel. All were from BD Biosciences, except where mentioned. For intracellular staining, BM cells were fixed with Lyse/Fix buffer, perforated with Perm Buffer III (both were from BD Biosciences), and stained with corresponding antibodies before being subjected to flow cytometry acquisition/analysis. Data were analyzed by FACSDIVA (BD Biosciences) or FlowJo 8.8.6 analysis software (TreeStar).
Quantitative RT-PCR analysis
Quantitative RT-PCR was performed using an iCycler iQ real-time PCR system (Bio-Rad) as described previously.23 Primers used for the quantitative assay are as follows: mFLT3-RT-5 forward, gctctgtctccccttcattg; mFLT3-RT-3 reverse, ccaggaccttcccaaactct; PU.1–5 forward, ATGCACGTCCTCGATACTCC; PU.1–3 reverse, GGCGAATCTTTTTCTTGCTG; GATA-1–5 forward, GAAGCGAATGATTGTCAGCA; and GATA-1–3 reverse, TTCCTCGTCTGGATTCCATC. The levels of transcripts were normalized to mS16.
BM transduction and transplantation
WT FLT3 (wtFLT3) cDNA was inserted under control of EF1α promoter in pWCC lentiviral vector. Lentiviral production and concentration were performed as described previously.25 After transduction, unsorted or sorted GFP+ cells were injected into the lateral tail vein of lethally irradiated (9 Gy) recipient mice (National Cancer Institute).
Results
Heterozygous (FLT3wt/ITD), hemizygous (FLT3−/ITD), and homozygous FLT3/ITD (FLT3ITD/ITD) mice develop progressive MPN
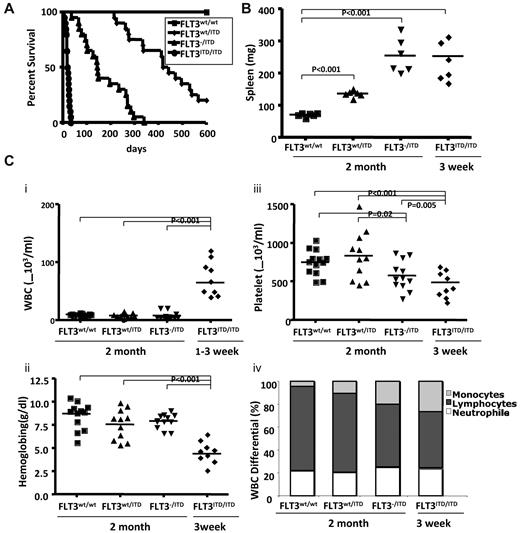
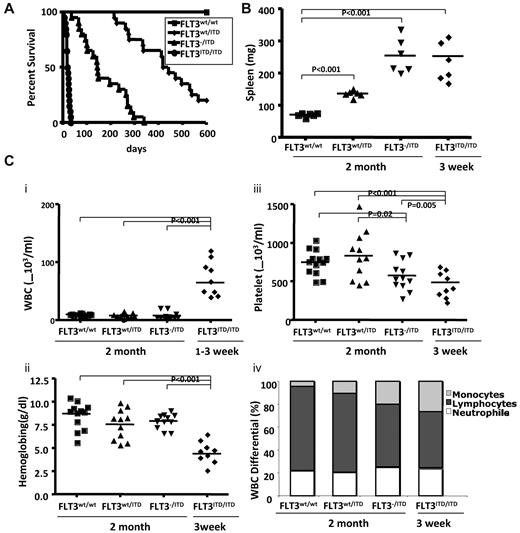
To investigate the roles of the FLT3 WT allele in disease induced by FLT3/ITD mutations, we generated hemizygous FLT3/ITD (FLT3−/ITD) mice by crossing FLT3wt/ITD with FLT3 knockout (FLT3−/−) mice. FLT3−/ITD mice were born viable with a birth rate consistent with the predicted Mendelian frequency. The median survival time for FLT3−/ITD mice was 145 days, considerably shorter than the more than 400-day median survival of FLT3wt/ITD mice. Homozygous (FLT3ITD/ITD) mice were generated by crossing FLT3wt/ITD mice with each other. The frequency of FLT3ITD/ITD newborn mice was approximately in accordance with the predicted Mendelian frequency, but most of them showed overt signs of disease by 2 to 3 weeks of age, characterized by smaller size, lethargy, hunched posture, and a pale and disheveled appearance. The median survival for FLT3ITD/ITD mice was approximately 25 days (Figure 1A).
FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice develop progressive, fatal MPN. (A) Kaplan-Meier plots (n = 20 for each group). (B) Spleen weights. (C) Peripheral WBC counts (i), hemoglobin (ii), platelet counts (iii), and WBC differential (iv) in 2-month-old FLT3wt/wt, FLT3wt/ITD, FLT3−/ITD, and in 3-week-old FLT3ITD/ITD mice
FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice develop progressive, fatal MPN. (A) Kaplan-Meier plots (n = 20 for each group). (B) Spleen weights. (C) Peripheral WBC counts (i), hemoglobin (ii), platelet counts (iii), and WBC differential (iv) in 2-month-old FLT3wt/wt, FLT3wt/ITD, FLT3−/ITD, and in 3-week-old FLT3ITD/ITD mice
FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice showed significantly more enlarged spleens, with 192 ± 56 mg and 255 ± 54 mg for 2-month-old FLT3wt/ITD, FLT3−/ITD, and 265 ± 67 mg for FLT3ITD/ITD mice at 3 weeks, respectively (Figure 1B), compared with 86 ± 22 mg for WT mice.
Compared with their WT littermates, 2-month-old FLT3wt/ITD mice had slightly lower peripheral WBC counts (8.3 ± 3.3 × 103/mL vs 10.1 ± 1.6 × 103/mL) but no differences in hemoglobin or platelet counts. FLT3−/ITD mice showed similar WBC counts (8.3 ± 6.5 × 103/mL for 2 months old) and hemoglobin levels but decreased platelet counts. FLT3ITD/ITD mice demonstrated extremely high WBC counts (85.2 ± 35.3 × 103/mL) by 3 weeks of age as well as extremely low hemoglobin levels and platelet counts (Figure 1Ci-iii). Differential counts showed that FLT3−/ITD and FLT3ITD/ITD mice had a trend toward a decreased fraction of lymphocyte counts and an increased fraction of neutrophil counts, but they were not of statistical significance. FLT3−/ITD mice showed a significantly increased fraction of monocytes in the peripheral blood. FLT3ITD/ITD mice demonstrated an even higher level of monocyte differential compared with the WT control or FLT3wt/ITD mice, indicating the possible role of FLT3/ITD mutations in monocytic expansion (Figure 1Civ).
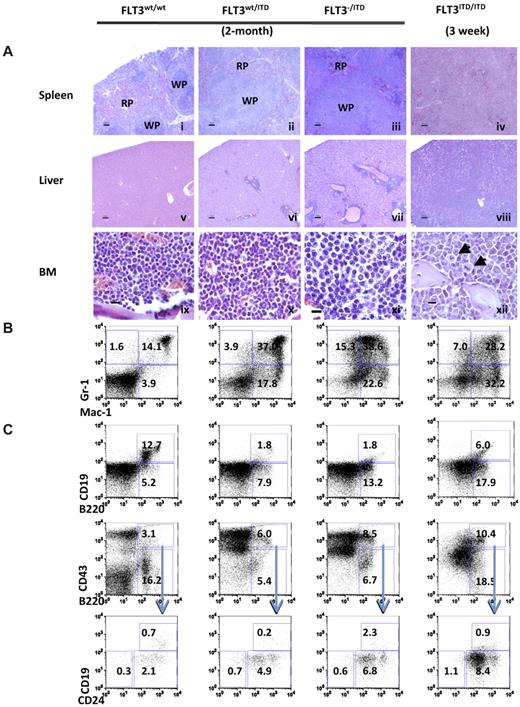
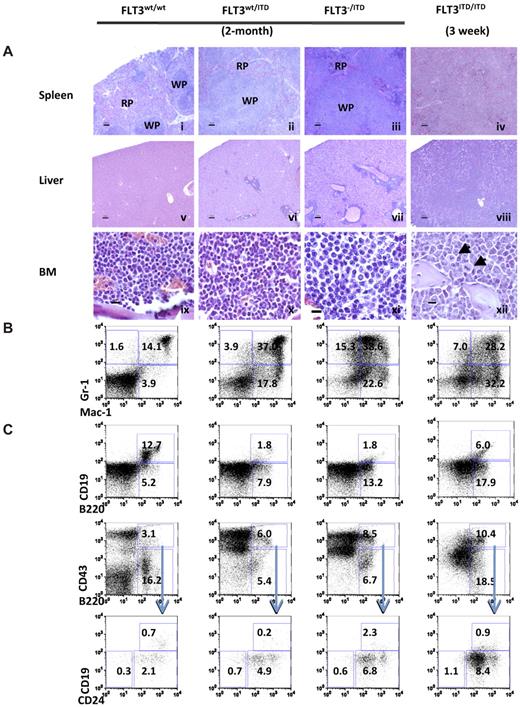
Hematoxylin and eosin stains demonstrated that spleen from 2-month-old FLT3wt/ITD mutants displayed notable myeloproliferative changes compared with the well-defined architecture of the WT mice, as evidenced by large areas of mature and immature myeloid-appearing cells in the red pulp. Two-month-old FLT3−/ITD mice demonstrated significant disruption of both the red and white pulp architecture of the spleen. Complete destruction of splenic architecture was also observed in FLT3ITD/ITD mice by 3 weeks of age (Figure 2Ai-iv). Extensive liver and other extramedullary tissue infiltration was observed in both FLT3−/ITD and FLT3ITD/ITD mice (Figure 2Av-viii). BM sections from 2-month-old FLT3wt/ITD mice demonstrated slightly increased cellularity, increased myeloid expansion, and reduced lymphoid composition, which were confirmed by differential counts of Wright-Giemsa-stained bone marrow cytospins (data not shown). FLT3−/ITD and FLT3ITD/ITD showed progressive expansion of myeloid cells compared with the FLT3wt/ITD mice and WT control of the same age (Figure 2Aix-xii). Some cells in FLT3ITD/ITD BM sections appear to carry aberrant mitoses, which might have resulted from uneven chromosome separation during cell division (Figure 2Axii arrowheads).
FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice develop progressive MPN. (A) Spleen (i-iv), liver (v-viii), and BM (ix-xii) from representative mice. (xii) Arrows indicate cells with aberrant mitoses. Scale bars are as follows: i-viii, 100 μm; ix-xii, 10 μm. Images for hematoxylin and eosin stain were acquired at room temperature using a Zeiss Axioskop upright microscope system (Carl Zeiss) with Achroplan 5×/0.16 NA, 10×/0.3 NA, and 40×/0.6 NA objectives and were photographed with an AxioCam camera (Carl Zeiss) and Axiovision Version 4.0 software (Carl Zeiss). Immunophenotype of BM from FLT3/ITD genotype mice demonstrates enhanced expansion of granulocytes/monocytes (B) and block of B-lymphoid development (C). Flow cytometric analysis of BM cells from representative 2-month-old FLT3wt/ITD, FLT3−/ITD mice, and a representative 3-week-old FLT3ITD/ITD mouse. Numbers indicate percentage of cells in whole BM.
FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice develop progressive MPN. (A) Spleen (i-iv), liver (v-viii), and BM (ix-xii) from representative mice. (xii) Arrows indicate cells with aberrant mitoses. Scale bars are as follows: i-viii, 100 μm; ix-xii, 10 μm. Images for hematoxylin and eosin stain were acquired at room temperature using a Zeiss Axioskop upright microscope system (Carl Zeiss) with Achroplan 5×/0.16 NA, 10×/0.3 NA, and 40×/0.6 NA objectives and were photographed with an AxioCam camera (Carl Zeiss) and Axiovision Version 4.0 software (Carl Zeiss). Immunophenotype of BM from FLT3/ITD genotype mice demonstrates enhanced expansion of granulocytes/monocytes (B) and block of B-lymphoid development (C). Flow cytometric analysis of BM cells from representative 2-month-old FLT3wt/ITD, FLT3−/ITD mice, and a representative 3-week-old FLT3ITD/ITD mouse. Numbers indicate percentage of cells in whole BM.
Flow cytometric analysis demonstrated that, compared with WT littermate controls, an increased fraction of BM cells from 2-month-old FLT3wt/ITD mice were positive for Mac-1 and/or Gr-1, indicating an expansion of the granulocytic/monocytic lineages (Figure 2B). There was a concomitant reduction of the megakaryocytic/erythrocytic lineages (data not shown) in FLT3wt/ITD mice. Compared with the FLT3wt/ITD mice, FLT3−/ITD mice have an even larger increased fraction of Mac-1+ and/or Gr-1+ fraction. Three-week-old FLT3ITD/ITD mice displayed higher Mac-1/Gr-1+ populations compared with the FLT3wt/ITD mice of the same age, but they were lower than the 2-month-old FLT3wt/ITD and FLT3−/ITD mice (data not shown). The increase in Mac-1+ and/or Gr-1+ BM cells over time is evidence that the myeloproliferative phenotype progresses with age. Because FLT3ITD/ITD mice died before 4 weeks of age, it was not possible to follow them to 2 months to see if the myeloid predominance progressed even further.
B-cell development was blocked at the early to late Pro-B transition in FLT3wt/ITD mice, as evidenced by the accumulation of CD93+B220+CD43+CD24+CD19− cells and the reduction in the fraction of B220+CD19+ cells.25 The accumulation of Pre-Pro-B cells was even more evident in FLT3−/ITD and FLT3ITD/ITD mice (Figure 2C). Three-week-old FLT3ITD/ITD mice also displayed an even bigger block in B-cell development compared with the FLT3wt/ITD and FLT3−/ITD mice of the same age (data not shown). Compared with the FLT3wt/ITD mice, both FLT3−/ITD and FLT3ITD/ITD mice displayed a higher fraction of B220+CD19low cells, which might be because of the “leaky” effect of some differentiation from the large pool of B220+CD43+ cells.
The results show that the FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice display increasingly progressive MPNs, respectively. The extent of MPNs appears to correlate with loss of the WT allele (FLT3wt/ITD vs FLT3−/ITD) and with the dose of mutant allele (FLT3−/ITD vs FLT3ITD/ITD). The increased aggressiveness of the MPNs in homozygous mice has been observed previously.22
FLT3−/ITD and FLT3ITD/ITD mice display a further increase in the fraction of primitive hematopoietic cells compared with FLT3wt/ITD mice
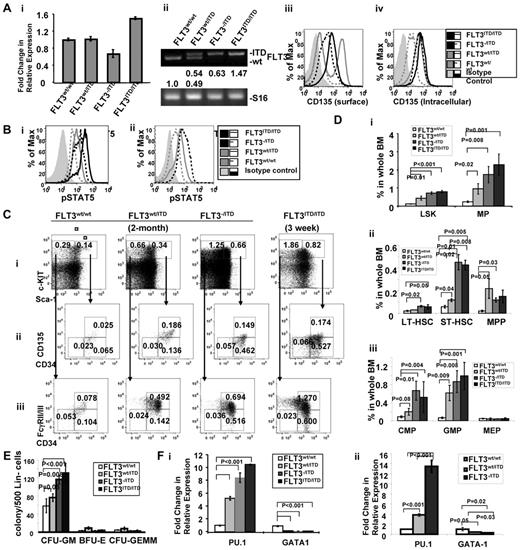
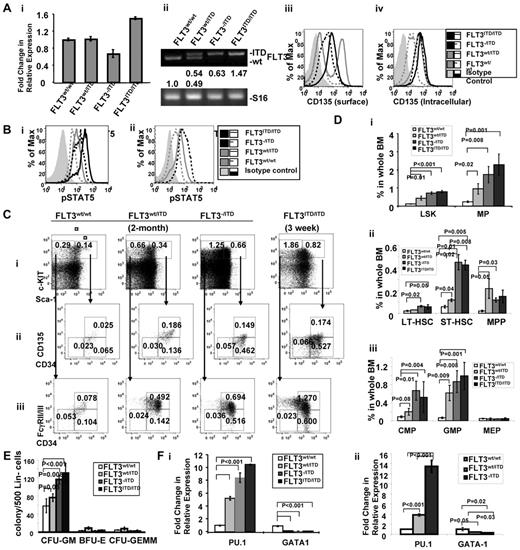
It appears that the relative expression level of FLT3/ITD influences the course of the disease in FLT3/ITD mice. We therefore examined the expression levels of the ITD versus WT allele in FLT3/ITD bone marrow. Quantitative RT-PCR demonstrated that total FLT3 expression in CD34+ multipotent progenitors (MPPs, Lin−Sca-1+c-KIT+CD34+), where FLT3 is known to be expressed at the highest level, in FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice are 1.02 ± 0.05-fold, 0.66 ± 0.09-fold, and 1.50 ± 0.03-fold of that of the WT littermates, respectively (Figure 3Ai). Higher levels of FLT3 expression in FLT3ITD/ITD cells might result from the increased expansion of FLT3+ cells in CD34+ MPPs. Densitometry analysis of the PCR products showed that relative expression of the ITD relative to the wt-FLT3 allele in FLT3wt/ITD mice was comparable (1.05 ± 0.07:1, Figure 3Aii). Flow cytometric analysis demonstrated reduced surface FLT3 expression in FLT3−/ITD and FLT3ITD/ITD BM. However, intracellular FLT3 expression in FLT3wt/ITD and FLT3−/ITD BM was comparable (Figure 3Aiii-iv).
Immature hematopoietic cells expand abnormally in FLT3/ITD mice. (A) Expression of FLT3 in BM from FLT3/ITD mice. (i) Quantitative RT-PCR analysis of FLT3 expression in CD34+ MPPs from FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice. (ii) Densitometry quantitation of RT-PCR products resulting from amplifying the WT and ITD alleles. Values below the electrophoresis image indicate relative fold changes of mRNA levels normalized to murine S16. Flow cytometry shows that surface FLT3 expression is reduced in FLT3−/ITD and FLT3ITD/ITD mice (iii), whereas intracellular expression of FLT3 in FLT3wt/ITD and FLT3−/ITD BM is comparable (iv). (Bii) Two- to 3-week-old FLT3ITD/ITD BM shows the highest level of phospho-STAT5 compared with the age-matched FLT3−/ITD, FLT3wt/ITD, and WT control. (ii) Two-month-old FLT3−/ITD and FLT3wt/ITD BM cells show increased levels of phospho-STAT5 compared with the WT control. (C-D) Flow cytometric analysis of BM from 2-month-old FLT3wt/ITD, FLT3−/ITD mice, and a 3-week-old FLT3ITD/ITD mouse. Numbers indicate percentages of cells in whole BM. Data were obtained from 5 individual mice in each group. (E) BM from FLT3−/ITD and FLT3ITD/ITD mice demonstrates enhanced granulocytic/monocytic colony-forming activity. Methylcellulose-based in vitro colony-forming assay of Lin− BM cells from 2-month-old WT, FLT3wt/ITD, FLT3−/ITD mice, and a 3-week-old FLT3ITD/ITD mouse. A representative of 3 experiments is shown. (F) Hematopoietic progenitors from FLT3/ITD BM have increased PU.1 and decreased GATA-1 expression. Quantitative RT-PCR using CD34+ MPP cells from 2- to 3-week-old WT, FLT3wt/ITD, and FLT3−/ITD, and FLT3ITD/ITD mice (i) or 2-month-old WT, FLT3wt/ITD, and FLT3−/ITD mice (ii). A representative of 3 experiments is shown. Data are expressed as mean ± SEM (error bars).
Immature hematopoietic cells expand abnormally in FLT3/ITD mice. (A) Expression of FLT3 in BM from FLT3/ITD mice. (i) Quantitative RT-PCR analysis of FLT3 expression in CD34+ MPPs from FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice. (ii) Densitometry quantitation of RT-PCR products resulting from amplifying the WT and ITD alleles. Values below the electrophoresis image indicate relative fold changes of mRNA levels normalized to murine S16. Flow cytometry shows that surface FLT3 expression is reduced in FLT3−/ITD and FLT3ITD/ITD mice (iii), whereas intracellular expression of FLT3 in FLT3wt/ITD and FLT3−/ITD BM is comparable (iv). (Bii) Two- to 3-week-old FLT3ITD/ITD BM shows the highest level of phospho-STAT5 compared with the age-matched FLT3−/ITD, FLT3wt/ITD, and WT control. (ii) Two-month-old FLT3−/ITD and FLT3wt/ITD BM cells show increased levels of phospho-STAT5 compared with the WT control. (C-D) Flow cytometric analysis of BM from 2-month-old FLT3wt/ITD, FLT3−/ITD mice, and a 3-week-old FLT3ITD/ITD mouse. Numbers indicate percentages of cells in whole BM. Data were obtained from 5 individual mice in each group. (E) BM from FLT3−/ITD and FLT3ITD/ITD mice demonstrates enhanced granulocytic/monocytic colony-forming activity. Methylcellulose-based in vitro colony-forming assay of Lin− BM cells from 2-month-old WT, FLT3wt/ITD, FLT3−/ITD mice, and a 3-week-old FLT3ITD/ITD mouse. A representative of 3 experiments is shown. (F) Hematopoietic progenitors from FLT3/ITD BM have increased PU.1 and decreased GATA-1 expression. Quantitative RT-PCR using CD34+ MPP cells from 2- to 3-week-old WT, FLT3wt/ITD, and FLT3−/ITD, and FLT3ITD/ITD mice (i) or 2-month-old WT, FLT3wt/ITD, and FLT3−/ITD mice (ii). A representative of 3 experiments is shown. Data are expressed as mean ± SEM (error bars).
It has been reported that FLT3/ITD mutation results in the activation of aberrant signaling pathways, such as STAT5 signaling.26 Constitutive activation of STAT5 and its downstream pathways may play a critical role in the leukemic transformation associated with FLT3/ITD. We are interested in understanding the differences in the downstream signaling pathways in FLT3/ITD cells. Intracellular phospho-STAT5 staining demonstrated that, in the lineage-negative BM cells, levels of phosho-STAT5 in FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice were increased compared with the WT control both in 2- to 3-week-old FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice (Figure 3Bi) and 2-month-old FLT3wt/ITD and FLT3−/ITD mice (Figure 3Bii). These results are consistent with the finding that FLT3/ITD, but not WT FLT3, significantly activates STAT5. The higher levels of STAT5 activation observed in FLT3ITD/ITD mice compared with the age-matched FLT3−/ITD mice also suggest that FLT3/ITD activates its abnormal downstream signaling in a dose-dependent manner.
In mice FLT3 is mainly expressed in normal hematopoietic stem/progenitor cells (HSPCs), especially MPPs (Lin−Sca-1+c-KIT+CD34+CD135+).27-29 Therefore, we wanted to examine the effect of FLT3 constitutive activation on HSPCs. An increased fraction of Lin−Sca-1+c-KIT+ cells (LSKs), characteristic of immature HSPCs, were detected in BM from 2-month-old FLT3wt/ITD mice (0.44% ± 0.11% in FLT3wt/ITD vs 0.12% ± 0.02% in WT control, n = 6, P = .01; Figure 3Ci,Di). Within this fraction, we detected slightly increased MPPs (0.22% ± 0.09% vs 0.02% ± 0.007%, P = .008) and short-term hematopoietic stem cells (ST-HSCs, Lin−Sca-1+c-KIT+CD34+CD135−, 0.12% ± 0.01% vs 0.06% ± 0.01%, P = .04) but not long-term hematopoietic stem cells (LT-HSCs, Lin−Sca-1+c-KIT+CD34−CD135−, 0.03% ± 0.008% vs 0.02% ± 0.004%, P = .07, Figure 3Cii,Dii). BM from FLT3−/ITD and FLT3ITD/ITD mice displayed an even higher fraction of LSKs (0.72% ± 0.07% with P < .001 for FLT3−/ITD mice and 0.83% ± 0.04% with P < .001 for FLT3ITD/ITD mice), ST-HSCs (0.45% ± 0.07% with P = .015 for FLT3−/ITD mice and 0.44% ± 0.08% with P = .005 for FLT3ITD/ITD mice) and LT-HSCs (0.06% ± 0.01% with P = .016 for FLT3−/ITD mice and 0.05% ± 0.04% with P = .047 for FLT3ITD/ITD mice) compared with WT mice. The fraction of MPPs was not further increased as might have been expected in FLT3−/ITD and FLT3ITD/ITD mice (0.12% ± 0.02% and 0.18% ± 0.05%, respectively, Figure 3Ci-ii,Di-ii) because of the reduced surface FLT3 expression and increased intracellular accumulation of FLT3 protein in them.
Further characterization showed that the populations of common myeloid progenitors (CMPs: Lin−Sca-1−c-KIT+CD34+FcγRII/III−; 0.20% ± 0.08% vs 0.08% ± 0.02% in WT littermates, n = 6, P = .02) and granulocytic/monocytic progenitors (GMPs: Lin−Sca-1−c-KIT+CD34+FcyRII/III+; 0.61% ± 0.17% vs 0.06% ± 0.02%, P = .009) were also expanded, whereas the fraction of megakaryocytic/erythrocytic progenitors (MEPs: Lin−Sca-1−c-KIT+CD34−FcγRII/III−; 0.04% ± 0.02% vs 0.05% ± 0.01%, P = .64) remained unchanged. The expansion of the CMP/GMP fractions was even more evident in FLT3−/ITD and FLT3ITD/ITD mice (0.67% ± 0.21% and 0.60% ± 0.15%, respectively, for CMP; 0.87% ± 0.24% and 1.07% ± 0.21%, respectively, for GMP; Figure 3Ciii,Diii). No obvious changes were again observed in the MEP fraction in either FLT3−/ITD or FLT3ITD/ITD BM, consistent with the finding that FLT3 plays roles in the expansion and differentiation of granulocytic/monocytic lineages but not megakaryocytic/erythrocytic lineages.27,28,30
In methylcellulose-based colony assays, lineage-depleted BM from FLT3wt/ITD mice demonstrated enhanced ability to generate CFU-GM. FLT3−/ITD and FLT3ITD/ITD BM generated further increases in CFU-GM compared with FLT3wt/ITD BM, whereas the numbers of BFU-E and CFU-GEMM were lower than those from the FLT3wt/ITD group (Figure 3E). The relative decrease in the reserve of BFU-E and CFU-GEMM might be because of the extreme expansion of myeloid progenitors, indicating that lineage-negative BM from FLT3−/ITD mice has higher ability to expand granulocytic/monocytic lineages compared with the FLT3wt/ITD mice.
We wanted to investigate the possible mechanisms for the expansion of GMPs and decreases in MEPs. The transcription factor network is known to play critical roles in hematopoietic lineage commitment.31,32 Recent studies have shown that the reciprocal activation of PU.1 and GATA-1 primarily organizes the early decisions of hematopoietic lineage commitment toward either myelolymphoid (granulocyte/monocyte/lymphoid-restricted) or myeloerythroid (megakaryocyte/erythroid) progenitor populations.33-36 Two independent populations, expressing either GATA-1 or PU.1 exclusively, reside within the CD34+ MPP fraction. Interestingly, 50% of CD34+ MPPs express surface FLT3 and a large fraction of FLT3− CD34+ MPPs express FLT3 transcripts despite undetectable cell surface FLT3.28,37
For these reasons, we assayed expression of PU.1 and GATA-1 in the CD34+ MPP population from FLT3/ITD BM using quantitative RT-PCR. The CD34+ MPP fraction from 2- to 3-week-old FLT3ITD/ITD mice displayed higher PU.1 expression compared with that from WT and FLT3wt/ITD mice (10.1 ± 0.08-fold, 2.28 ± 0.02-fold, and 1.05 ± 0.01-fold of that of WT, FLT3wt/ITD, and FLT3−/ITD mice, respectively: P < .001, P < .001, and P = .30, respectively, n = 3). Likewise, decreased GATA-1 expression was also observed in CD34+ MPP cells from FLT3ITD/ITD mice compared with the age-matched WT and FLT3wt/ITD mice (0.06 ± 0.01-fold, 0.57 ± 0.08-fold, and 0.93 ± 0.18-fold compared with that of WT, FLT3wt/ITD, and FLT3−/ITD mice, respectively: P < .001, P = .003, and P = .16, respectively, n = 3; Figure 3Fi). Similarly, PU.1 expression was increased in CD34+ MPP cells from FLT3wt/ITD and even further in FLT3−/ITD BM from 2-month-old mice (3.82 ± 0.27-fold and 13.47 ± 1.16-fold, respectively, compared with CD34+ MPP cells in WT mice, P < .001 for both, n = 3). In contrast, expression of GATA-1 was significantly deceased in CD34+ MPP cells from both FLT3wt/ITD and FLT3−/ITD BM (0.44 ± 0.08-fold and 0.29 ± 0.02-fold, respectively; P = .05 and .02, respectively, n = 3; Figure 3Fii).
These results suggest that, in CD34+ MPPs, constitutively activated FLT3 might increase PU.1 expression and simultaneously decrease GATA-1 expression, resulting in the subsequent expansion of granulocytic/monocytic/lymphocytic progenitors (GMLPs) and a decrease in MEPs.
Fully transformed leukemia developed in some of the FLT3ITD/ITD, but not FLT3wt/ITD or FLT3−/ITD mice
In the initial knockin mice we generated, the presence of the PGK-Neo cassette downstream of the FLT3 promoter (which is used for ES clone screening) reduced transcription of the FLT3/ITD allele to a level of 30%-40% of the WT allele (data not shown). For the ITD allele to be expressed at the same level as its WT counterpart, we used CMV-Cre transgenic mice to excise the LoxP-flanked PGK-Neo cassette. Transcription of the floxed ITD allele increases to the level of the WT allele, and these are the mice that were used in the characterization study. However, PGK-Neo-excised FLT3ITD/ITD mice die of fatal MPN at a very young age (before 4 weeks), making it impossible to follow them long enough for the development of any spontaneous leukemia. Compared with the PGK-Neo-excised FLT3ITD/ITD mice, FLT3ITD/ITD mice carrying PGK-Neo cassette survived longer, which allowed us to monitor the development of spontaneous leukemia in these mice. These mice died within 2 to 14 months of age, with most of the mice showing overt signs of disease characterized by smaller size, slow movement, hunchback, and a disheveled appearance.
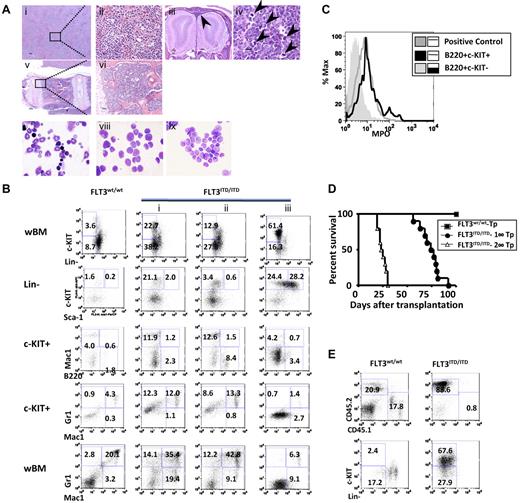
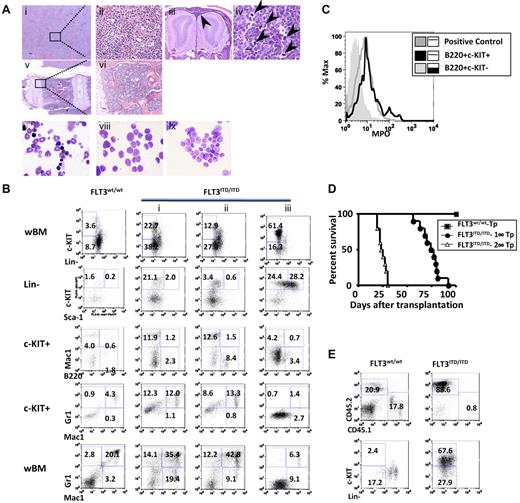
A small percentage (7%, 9 of 129) of the PGK-Neo+ FLT3ITD/ITD mice spontaneously developed fully transformed leukemia, with latency ranging from 139 to 304 days. The diagnosis was made based on the Bethesda criteria for nonlymphoid leukemia.38 Histopathologic analysis revealed complete effacement of the normal splenic architecture, with extensive infiltration of immature myeloid cells in tissues, including parenchyma and sinusoids of the liver (Figure 4Ai-ii) and meninges (Figure 4Aiii). Enlarged lymph nodes were found in some of the leukemic mice, with architecture effaced by sheets of homogeneous-appearing cells and numerous aberrant mitotic figures (Figure 4Aiv arrowheads). BM was hypercellular and completely replaced by immature cells (Figure 4Av-vi). Wright-Giemsa stain of BM cytospins showed frequent blasts compared with WT littermates (Figure 4Avii-viii).
Some of the FLT3ITD/ITD mice develop leukemia, which is transplantable. (A) Leukemia in an FLT3ITD/ITD mouse. (i-ii) Liver with significant leukemic infiltration. (iii) Olfactory bulbs of the brain surrounded by infiltrating tumor cells in the meninges (arrowhead). (iv) Lymph node with architecture effaced by sheets of blasts and numerous aberrant mitotic figures (arrowheads). (v-vi) Vertebral BM replaced by sheets of leukemic blasts. (vii-ix) Wright-Giemsa stain of BM cytopsins demonstrates increased fraction of blasts in a leukemic FLT3ITD/ITD mouse (viii) and its recipients after BM transplantation (ix) compared with a WT control (vii). Scale bars are as follows: iii, 200 μm; i,v, 100 μm; and ii,iv,vi, 10 μm. Image acquisition for hematoxylin and eosin stain is the same as described in Figure 2. Images for Wright-Giemsa stain were acquired using a Nikon Eclipse E600 microscope system with a Nikon 100×/0.90 NA oil objective (original magnification ×1000) and were photographed with a Nikon DMX 1200 digital camera with ACT-1 2.0 software (Nikon). (B) Flow cytometric analysis demonstrates increased immature fraction in representative leukemic FLT3ITD/ITD mice. (C) B220+c-KIT+, but not B220+c-KIT− cells, from FLT3ITD/ITD leukemic mice are myelperoxidase positive. Mac-1+/Gr-1+ cells from a WT mouse were used as positive control. (D) Kaplan-Meier plot of the survival of recipients after the first (1°-Tp) and secondary (2°-Tp) transplantation of leukemic FLT3ITD/ITD BM cells. (E) Flow cytometric analysis shows the accumulation of immature cells in BM from a representative recipient mouse transplanted with leukemic FLT3ITD/ITD cells. Numbers indicate percentage of cells in whole BM.
Some of the FLT3ITD/ITD mice develop leukemia, which is transplantable. (A) Leukemia in an FLT3ITD/ITD mouse. (i-ii) Liver with significant leukemic infiltration. (iii) Olfactory bulbs of the brain surrounded by infiltrating tumor cells in the meninges (arrowhead). (iv) Lymph node with architecture effaced by sheets of blasts and numerous aberrant mitotic figures (arrowheads). (v-vi) Vertebral BM replaced by sheets of leukemic blasts. (vii-ix) Wright-Giemsa stain of BM cytopsins demonstrates increased fraction of blasts in a leukemic FLT3ITD/ITD mouse (viii) and its recipients after BM transplantation (ix) compared with a WT control (vii). Scale bars are as follows: iii, 200 μm; i,v, 100 μm; and ii,iv,vi, 10 μm. Image acquisition for hematoxylin and eosin stain is the same as described in Figure 2. Images for Wright-Giemsa stain were acquired using a Nikon Eclipse E600 microscope system with a Nikon 100×/0.90 NA oil objective (original magnification ×1000) and were photographed with a Nikon DMX 1200 digital camera with ACT-1 2.0 software (Nikon). (B) Flow cytometric analysis demonstrates increased immature fraction in representative leukemic FLT3ITD/ITD mice. (C) B220+c-KIT+, but not B220+c-KIT− cells, from FLT3ITD/ITD leukemic mice are myelperoxidase positive. Mac-1+/Gr-1+ cells from a WT mouse were used as positive control. (D) Kaplan-Meier plot of the survival of recipients after the first (1°-Tp) and secondary (2°-Tp) transplantation of leukemic FLT3ITD/ITD BM cells. (E) Flow cytometric analysis shows the accumulation of immature cells in BM from a representative recipient mouse transplanted with leukemic FLT3ITD/ITD cells. Numbers indicate percentage of cells in whole BM.
Compared with FLT3ITD/ITD mice with fatal MPN, flow cytometric analysis of the leukemic mice showed a higher fraction of c-Kit+ (> 20%) and/or Lin−/low cells, with an expansion of the myeloid progenitors (Lin−Sca-1−c-KIT+). The fraction of Mac-1+ and/or Gr-1+ cells was increased in 6 of 9 cases, mimicking French-American-British classification M2 in human AML (eg, Figure 4Bi). In 2 other cases, a moderate increase in B220+c-KIT+ cells was observed (eg, Figure 4Bii). This fraction of cells was transplantable and was positive for myelperoxidase, a key functional marker for myeloid lineage (Figure 4C), suggesting that they were AML with minimal differentiation. One of the leukemic mice showed a large expansion of the LSK fraction with extremely low levels of Mac-1+ or Gr-1+ cells (Figure 4Biii), indicative of the involvement of an even more primitive compartment in leukemia development. No leukemia of erythrocytic or megakaryocytic origin has been observed in these mice, consistent with the finding that FLT3 expression plays roles in the differentiation and expansion of granulocytic/monocytic but not megakaryocytic/erythrocytic lineages.27,28,30
Injection of 1 × 106 total spleen or BM cells from the leukemic mice into syngeneic recipients resulted in the development of leukemia in recipients with a latency of 2 to 3 months, characterized by leukocytosis, splenomegaly, and an increased fraction of c-KIT+ cells, phenotypes similar to the leukemic donors (Figure 4D-E). Retransplantation of the same number of BM cells from recipients resulted in the development of the same type of leukemia in secondary recipients within 4 weeks (Figure 4Aix,E). No signs of fully transformed leukemia were observed in FLT3wt/ITD or FLT3−/ITD mice over time, suggesting that the loss of WT allele and a higher dose of constitutively activated FLT3 contribute to the onset of leukemia in FLT3ITD/ITD mice.
Introducing WT FLT3 into FLT3−/ITD BM slows the development of MPN in recipient mice
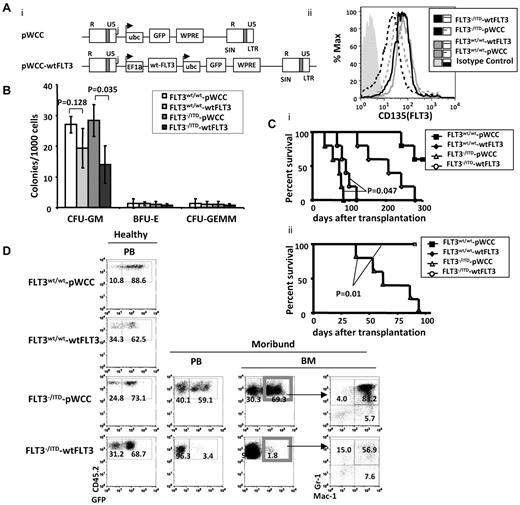
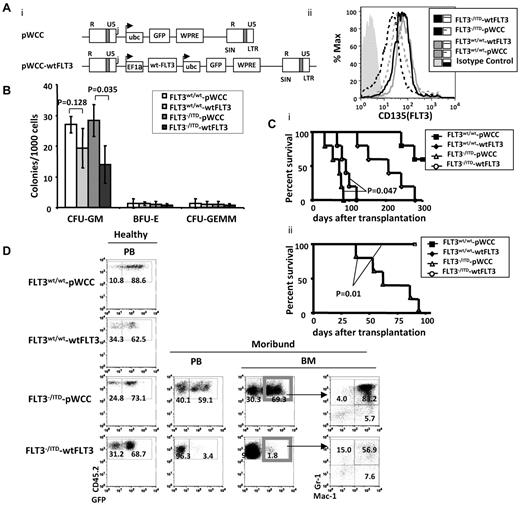
To further explore the potential moderating effect of wtFLT3 expression on FLT3/ITD-associated MPNs, we transduced wtFLT3 or pWCC, a lentiviral vector control (each coexpressing GFP), into lineage-depleted FLT3−/ITD CD45.2 BM cells and cultured the cells in vitro or injected them into CD45.1 recipients (Figure 5Ai). Flow cytometric analysis showed that transduction of the FLT3−/ITD BM with the wtFLT3 construct increased FLT3 expression to levels comparable with that of WT BM in the GFP+ cells (Figure 5Aii).
Replacement of the FLT3wt/wt in FLT3−/ITD BM postponed the development of fatal MPN in recipient mice. (A) Structure of the dual-promoter wtFLT3 lentiviral vector used in these studies (i) and expression of FLT3 in GFP+ transduced cells (ii). Transcripts from lentivirus with single or dual internal promoters are denoted with arrows. R and U5 indicates the repeat and U5 region, respectively, in the viral LTR; WPRE, woodchuck post-transcriptional regulatory element; EF1α, human EF1α promoter/enhancer; Ubc, human ubiquitin promoter; and GFP, enhanced green fluorescent protein. (B) Methylcellulose-based in vitro colony-forming assay was performed on GFP+ lentiviral-transduced lineage-depleted BM from 2-month-old mice. (C) Kaplan-Meier survival curve for recipients of viral-transduced unsorted (i) and sorted (ii) FLT3−/ITD Lin− cells. (D) Flow cytometric analysis of BM from recipients of unselected transduced BM. Numbers indicate the percentages of cells in whole BM.
Replacement of the FLT3wt/wt in FLT3−/ITD BM postponed the development of fatal MPN in recipient mice. (A) Structure of the dual-promoter wtFLT3 lentiviral vector used in these studies (i) and expression of FLT3 in GFP+ transduced cells (ii). Transcripts from lentivirus with single or dual internal promoters are denoted with arrows. R and U5 indicates the repeat and U5 region, respectively, in the viral LTR; WPRE, woodchuck post-transcriptional regulatory element; EF1α, human EF1α promoter/enhancer; Ubc, human ubiquitin promoter; and GFP, enhanced green fluorescent protein. (B) Methylcellulose-based in vitro colony-forming assay was performed on GFP+ lentiviral-transduced lineage-depleted BM from 2-month-old mice. (C) Kaplan-Meier survival curve for recipients of viral-transduced unsorted (i) and sorted (ii) FLT3−/ITD Lin− cells. (D) Flow cytometric analysis of BM from recipients of unselected transduced BM. Numbers indicate the percentages of cells in whole BM.
Methylcellulose assay of sorted GFP+ cells demonstrated a significant reduction in the generation of CFU-GM colonies for wtFLT3-transduced FLT3−/ITD BM compared with the pWCC vector-transduced FLT3−/ITD BM (Figure 5B). No significant changes in the numbers of BFU-E or CFU-GEMM colonies were observed. It is notable that the numbers of BFU-E and CFU-GEMM colonies generated were low, which might make it difficult to discern subtle colony number differences. These results suggest that FLT3 signaling mostly affects the expansion of granulocytic/monocytic colonies and is consistent with the known role of normal FLT3 signaling in myeloid proliferation.28-30
Recipients injected with unselected pWCC-transduced FLT3−/ITD BM started to die 36 days after injection and the median survival for the group was 73 days. In contrast, the group of recipients injected with unselected wtFLT3-transduced FLT3−/ITD BM did not have their first death until 67 days after transplantation and had a longer median survival of 91 days (P = .047 compared with the pWCC-transduced FLT3−/ITD group, log-rank test, n = 5 for each group, Figure 5Ci). Recipients of unselected wtFLT3-transduced WT BM died with a median survival of 203 days, and recipients of unselected pWCC-transduced WT BM showed death at ∼ 250 days. The late deaths in the WT transduced groups might be a result of retroviral insertional mutagenesis and/or toxicities associated with the retroviral infection and/or transplant.
BM from the dying pWCC-transduced FLT3−/ITD recipients was hypercellular. FACS analysis demonstrated that both the CD45.2+GFP+ and CD45.2+GFP− (data not shown) cells had an increased fraction of Mac-1+/Gr-1+ cells, consistent with MPN of the donors. In contrast, the CD45.2+GFP+ BM cells from wtFLT3-transduced FLT3−/ITD BM showed a reduced fraction of Mac-1+/Gr1+ cells, suggesting a moderated level of MPNs (Figure 5D). In these experiments, we had injected the unsorted lentiviral-transduced Lin−BM into recipient mice. The initial percentage of GFP+ cells in the unsorted pWCC and wtFLT3-transduced Lin− FLT3−/ITD BM before transplantation were 68% and 57%, respectively. Interestingly, further analysis of BM from sick wtFLT3-transduced FLT3−/ITD recipients demonstrated that the BM was composed of 99% GFP− cells (Figure 5D), suggesting a proliferative advantage for the FLT3−/ITD Lin− cells that had not been successfully transduced with WT FLT3. These results suggest that the presence of WT FLT3 delays and moderates the development of MPN caused by FLT3/ITD mutations.
The presence and extreme expansion of the untransduced wtFLT3− (GFP−) cells in the wtFLT3-trasduced FLT3−/ITD BM recipients might account for the fatal MPNs and somewhat shorten the survival of the recipients. Therefore, we sorted and transplanted GFP+ BM to examine the phenotype of the MPNs in the recipients of successfully transduced BM. We found that the pWCC-transduced GFP+FLT3−/ITD recipients died early with a median survival of 62 days, comparable with the outcome of the unsorted-transplant recipients. In contrast to the previous small increase in median survival (73-91 days), all of the wtFLT3-transduced GFP+FLT3−/ITD recipients still appeared healthy at 100 days, after the last of the pWCC-transduced GFP+FLT3−/ITD recipients has died of fatal MPN. The log-rank test showed a highly significant survival difference between the 2 groups, with a P value of .01 (Figure 5Cii).
In summary, our results showed that FLT3wt/ITD, FLT3−/ITD, and FLT3ITD/ITD mice developed progressive MPNs. The FLT3−/ITD and FLT3ITD/ITD mice showed a more severe phenotype of MPN. The introduction of WT FLT3 delayed the development of MPNs caused by FLT3/ITD mutation. These results suggest that loss of WT allele contributes to ITD-related myeloid expansion, thus appearing to attenuate the function of the FLT3/ITD in these mice.
Discussion
The generation of homozygous and hemizygous FLT3/ITD mice enabled us to study the interactive roles WT FLT3 and FLT3/ITD mutations play in the development of MPN/leukemia. It has been previously reported, in a FLT3/ITD knockin mouse model, that the extent of myeloid expansion and block of B-cell development in FLT3wt/ITD and FLT3ITD/ITD mice correlate with the dose of the ITD allele.22 The number, survival, and cell-cycle status of multipotent stem and progenitor cells in these mice also correlate with the dose of the ITD allele. Consistent with this, we also observed more extensive phenotype in the FLT3ITD/ITD versus the FLT3wt/ITD mice. Of even greater interest was the finding that FLT3−/ITD mice, which have lost the WT allele, displayed a worse MPN phenotype than the FLT3wt/ITD mice. The demonstration of reduced MPN in the FLT3−/ITD mice, in which the wtFLT3 allele was transduced back, confirms the suppressive effect the WT allele plays in myeloid disease involving FLT3/ITD signaling.
WT FLT3 receptor is localized primarily to the cell surface, whereas FLT3/ITD mutants are retained as a high-mannose precursor in certain intracellular compartment(s), most likely endoplasmic reticulum.39,40 ER-anchored FLT3/ITD retains STAT5-activating capacity and can transform cells in vitro and in vivo.40 In the FLT3/ITD mice, FLT3−/ITD and FLT3ITD/ITD cells show lower surface but higher intracellular FLT3 expression compared with FLT3wt/ITD cells. The intracellular accumulation of FLT3 protein in FLT3−/ITD and FLT3ITD/ITD cells could be the result of the increased FLT3/ITD activity and the lack of WT FLT3 expression.
The MPP population has been shown to contain a fraction of cells called earliest lymphoid progenitors, which differentiates into GMLPs but lacks megakaryocytic/erythrocytic lineage potential.41 Similarly, FLT3+ MPP cells have been shown to be primed for lymphoid lineage development (lymphoid-primed multipotent progenitor [LMPP]).28 These LMPPs also have the ability to differentiate into granulocytic/monocytic lineages but not megakaryocytic/erythrocytic lineages. It has been proposed that GMPs can be generated from both the FLT3+ MPPs and CMP.33 In the FLT3/ITD knockin mouse model, in which FLT3 is constitutively activated in HSPCs, we found the accumulation of LMPPs, CLPs (data not shown), CMPs, and GMPs, but not MEPs. Subsequently, these mice had increased early B-cell progenitors, granulocytes, and monocytes but decreased megakaryocytes/erythrocytes. The findings thus provide evidence that activated FLT3 causes expansion of progenitors other than MEPs. It supports the aforementioned hematopoietic developmental pathway model, in which FLT3+ MPPs give rise to GMLPs but not MEPs.28,33,41
In the FLT3/ITD knockin mouse model, we detected an increased fraction of CMP and GMP cells but not MEP cells. The reciprocal activation of PU.1 and GATA-1 exerts critical instructive signals for GM and ME lineage commitment, respectively.19,31,33,35,36 PU.1 transactivates a series of GM- and lymphoid-related genes and is indispensable for development of CMPs, GMPs, and CLPs. In contrast, GATA-1 is an essential transcription factor for the megakaryocytic/erythrocytic lineages.19,33,35,36 The antagonistic interplay of PU.1 and GATA-1 is found to play a critical role in early hematopoietic fate decision, such as the GM or lymphoid versus the megakaryocytic/erythrocytic lineages.42 Using PU.1-GFP and GATA-1-GFP reporter mouse models, it was observed that PU.1-GFP is expressed in FLT3+ MPPs. In contrast, GATA-1-GFP+ cells are only found in FLT3− MPPs.33 Consistent with this, in our study, we observed increased expression of PU.1 in CD34+ MPPs from FLT3/ITD BM, and decreased expression of GATA-1 in the same population, supporting the reciprocal and competitive roles of PU.1 and GATA-1 in progenitor lineage commitment. Increased PU.1 and decreased GATA-1 expression in CD34+ MPPs could be explained by either the expanded FLT3+ fraction within the MPP compartment or by FLT3 activation in individual FLT3+ MPP cells. We further found increased PU.1 and decreased GATA-1 in the FLT3+CD34+ MPP (LMPPs), suggesting that both the expansion of FLT3+ MPPs and the constitutive activation of FLT3 in individual FLT3+ MPP cells contribute to the overall reciprocal changes in expression of PU.1 and its antagonistic counterpart GATA-1, with a subsequent accumulation of lymphoid and myeloid progenitors other than MEPs. These findings suggest that constitutively activated FLT3 causes the expansion of GM and lymphoid progenitors through elevated expression of PU.1 and simultaneous reduction of its competitor, GATA-1.
Homozygous mice, with the loss of the WT allele and the addition of one more ITD allele, displayed the most severe myeloid expansion. Interestingly, FLT3ITD/ITD mice carrying a PGK-Neo cassette within the ITD alleles, and therefore expressing a lower level of FLT3/ITD transcription, developed relatively mild MPNs compared with the FLT3ITD/ITD mice with the PGK-Neo cassette floxed. This suggests that the level of FLT3 expression, or FLT3 activation, also affects the extent of myeloid expansion. Previously, we and others demonstrated that FLT3/ITD mutations increase reactive oxygen species production which leads to increased DNA double strand breaks and decreased repair fidelity with the accumulation of additional mutations as a consequence.43,44 This may in part explain the aggressive nature of AML in FLT3/ITD patients. In this study, fully transformed leukemia developed in a fraction of the homozygous FLT3/ITD mice but not the heterozygous or even the hemizygous FLT3/ITD mice. It is possible that higher FLT3/ITD activity causes a higher level of genomic instability and the possible acquisition of additional mutations. As a result, some of the homozygous mice are able to develop spontaneous leukemia quickly enough to be detected before they are already destined to die of fatal MPN. Interestingly, all of the hemizygous FLT3/ITD mice died of MPN. No acute leukemia was observed in these mice. With a lower FLT3/ITD activity compared with the homozygous FLT3/ITD mice, FLT3/ITD hemizygous mice might die of fatal MPN before their BM accumulates additional “hits” to develop into full-blown leukemia.
Our model shows that FLT3/ITD mutations confer myeloid proliferation. Appropriate expression of WT FLT3 appears to modulate the myeloid expansion caused by FLT3/ITD mutations. The level of FLT3/ITD expression also affects the extent of myeloid expansion. The association between the ratio of ITD versus WT expression and the consequent phenotypic differences in the disease models described here may reflect differences in the downstream targets activated by FLT3/ITD and WT FLT3. These models will provide a good tool to further study the pathways by which FLT3/ITD signaling contributes to leukemogenesis and to further understand the extremely poor prognosis of patients with FLT3/ITD mutations and LOH.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the D.S. laboratory for their insightful discussions and valuable comments on the manuscript.
This work was supported by the National Cancer Institute (grants CA90668 and CA70970) and the Giant Food Pediatric Cancer Research Fund. D.S. is also supported by the Kyle Haydock Professorship.
National Institutes of Health
Authorship
Contribution: L.L. designed and performed experiments, analyzed data, and wrote the first draft; E.B. and S.G. designed and performed experiments; D.H. performed experiments and analyzed data; and D.S. designed experiments, analyzed data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald Small, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, CRB Rm 251, 1650 Orleans St, Baltimore, MD 21231; e-mail: donsmall@jhmi.edu.