Abstract
Immune thrombocytopenia (ITP) is an autoimmune disease with a complex pathogenesis. As in many B cell–related autoimmune diseases, rituximab (RTX) has been shown to increase platelet counts in some ITP patients. From an immunologic standpoint, the mode of action of RTX and the reasons underlying its limited efficacy have yet to be elucidated. Because splenectomy is a cornerstone treatment of ITP, the immune effect of RTX on this major secondary lymphoid organ was investigated in 18 spleens removed from ITP patients who were treated or not with RTX. Spleens from ITP individuals had follicular hyperplasia consistent with secondary follicles. RTX therapy resulted in complete B-cell depletion in the blood and a significant reduction in splenic B cells, but these patients did not achieve remission. Moreover, whereas the percentage of circulating regulatory T cells (Tregs) was similar to that in controls, splenic Tregs were reduced in ITP patients. Interestingly, the ratio of proinflammatory Th1 cells to suppressive Tregs was increased in the spleens of patients who failed RTX therapy. These results indicate that although B cells are involved in ITP pathogenesis, RTX-induced total B-cell depletion is not correlated with its therapeutic effects, which suggests additional immune-mediated mechanisms of action of this drug.
Introduction
Immune thrombocytopenia (ITP) is a rare autoimmune disease with an incidence of 2.6-6.6 per 100 000 adults per year.1,2 It is characterized by a low platelet count that may result in bleeding and a mortality rate of 1%-4% per year.3 The pathogenesis of ITP, involving both the immune peripheral destruction of platelets and insufficient BM production, is complex and is still not completely understood.4 Although it is not pathognomonic, because it can also be observed in other pathologic conditions, antiplatelet antibodies against GPIIb/IIIa are often detected in ITP.5 Opsonized platelets are preferentially eliminated in the spleen by macrophages. Antiplatelet antibodies also trigger complement-dependent cytotoxicity6 and induce apoptosis of platelet progenitors, megakaryocytes, which express GPIIb/IIIa.7 Cytotoxic T lymphocytes may also play a part in the peripheral destruction of platelets and in megakaryocyte destruction, because their recruitment in BM is increased in ITP.8,9 Antiplatelet auto-antibodies are usually isotype switched and harbor somatic mutations consistent with cooperation with T cells10 and with the Th1 cytokine profile associated with ITP.11,12 Conflicting results have been reported on the role of immunosuppressive regulatory T cells (Tregs) in ITP.13 Reduced immunosuppressive function of Tregs in ITP has been reported,14-17 and various therapies that restore platelet counts, such as rituximab16 and thrombopoietic agents,14 have been reported to normalize Treg function. However, there is still controversy regarding the number of circulating Tregs in ITP. Some studies have documented a decrease in the number of peripheral Tregs,15,16,18,19 whereas other reports have suggested that Treg frequency remains unchanged in ITP patients.14,17
Different strategies have been considered for the treatment of ITP. Steroids are the first-line therapy and result in systemic and profound immunosuppression. Intravenous immunoglobulin (IVIg) and Rhesus D antibodies compete with Fcγ receptors on macrophages and thus lead to a transient response. Surgical splenectomy results in the elimination of splenic macrophages and possibly corrects the immune dysregulation associated with ITP. Immunosuppressive drugs that negatively target both T and B lymphocytes are also being used. Additional new drugs known as thrombopoietin mimetics trigger an increase in BM platelet production. As in many other autoimmune diseases involving B lymphocytes,20 rituximab (RTX) therapy is efficient in ITP, with response rates of 40%, 33%, and 21% at 1, 2, and 5 years of follow-up, respectively.21,22 RTX is a chimeric mAb targeting CD20, a molecule expressed by the B-cell lineage (except pro-B cells and plasma cells). Binding of RTX to CD20 triggers lymphocyte depletion by different mechanisms, including apoptosis, complement-dependent cytotoxicity, and antibody-dependent cytotoxicity.20,23 After RTX administration, the rapid and complete depletion of circulating B cells is observed. However, the mechanism of action of RTX on immune cells in ITP has not yet been completely elucidated. Evidence has been provided that RTX may not only target B cells, but may also act on circulating helper T (Th) cells and cytotoxic T (Tc) cells, which may normalize the Th1/Th2 and Tc1/Tc2 ratios, which are commonly increased in ITP patients.24
Although the spleen is one of the main secondary lymphoid organs, few studies have characterized splenic immune responses and the modulation of these responses in human autoimmune diseases.25,26 In addition, the influence of RTX on B and Th cells in secondary lymphoid organs has never been investigated in humans except in 2 isolated cases.27 As splenectomy is the second-line treatment in ITP, with a response rate of 66%.28 This therapeutic approach presents a very rare clinical opportunity to study splenic cells in human autoimmune diseases. The current study was performed to investigate splenic immune responses in ITP patients and to address the immunomodulatory effects of RTX.
Methods
Patients
Forty ITP patients admitted to the University Hospital of Dijon, France were enrolled in this study after they gave a written informed consent in accordance with the Declaration of Helsinki. The study was approved by the institutional review board and the ethics committee of the University Hospital of Dijon. The main inclusion criterion was thrombocytopenia (platelet count < 100 × 109/L). Familial, viral, or drug-induced etiologies were excluded. Patients suffering from other autoimmune diseases (eg, systemic lupus erythematosus and antiphospholipid syndrome) were also excluded. Most of the patients were treated with steroids for 3 weeks and, if necessary, with IVIg as first-line therapies, followed by dapsone. Rituximab and splenectomy were used as the second-line therapy after 6 months and 1 year of follow-up, respectively. Blood samples were obtained at diagnosis and 1 month after rituximab infusion when needed. Response rates were defined as follows: complete response when the platelet count was > 100 × 109/L, partial response when the platelet count ranged between 30 and 100 × 109/L with at least a 2-fold increase in the initial platelet count, and no response when the platelet count was < 30 × 109/L. Patient characteristics are reported in Table 1. Twenty-eight sex- and age-matched healthy donors were used as controls. Blood samples obtained from 27 ITP patients were available at diagnosis. The different lymphocyte subsets (CD4+ T cells, CD8+ T cells, B cells, and natural killer cells) were not significantly different (Table 2). The spleens of 18 ITP patients were available for histologic, flow cytometry (FCM), and RT-PCR analyses. The characteristics of ITP patients who underwent splenectomy are reported in Table 3. Three of the 10 patients who were treated with RTX received a 4-infusion regimen (375 mg/m2 weekly for 4 weeks) corresponding to a mean total dose of 2.21 ± 0.37 g. The 7 remaining patients were treated using a 2-dose regimen (1 g on days 1 and 15), representing a mean total dose of 2 g. Seven spleens obtained from posttrauma splenectomies were used as controls. All but one of these controls received RBC transfusions before splenectomy.
Spleen preparation
The spleens were obtained from ITP patients during scheduled surgery within the hour of their removal. Posttrauma spleens were stored from 2-4 hours at 4°C until processing. Briefly, sterile spleen tissues were mechanically disrupted using a syringe plunger. After the cells were dissociated, they were filtered through a 100-μm nylon strainer. Cell suspension was incubated for 10 minutes in a hemolytic solution (150mM ammonium chloride, 10mM potassium bicarbonate, and 0.1mM EDTA) at room temperature to remove RBCs. Cells were then washed in medium (RPMI with 10% FBS) and filtered again. Cell viability was assessed using trypan blue exclusion and was generally > 90%. Samples were then divided for FCM analysis, RNA extraction, and CD4 isolation. Remaining cells were stored in liquid nitrogen.
Antibodies
The following antibodies were used for FCM analysis of lymphocyte subsets: anti-CD3 FITC, anti-CD4 PE, anti-CD8 PE-Cy5, anti-CD16+CD56 PE, anti-CD19 PE-Cy5, anti-kappa FITC, anti-lambda PE, anti-CD45 PE-Cy7 (Beckman Coulter), anti-CD4 APC, anti-CD8 PB, anti-CD19 APC, and anti-CD27 PE-Cy7 (eBioscience). Treg staining was performed with anti-CD4 PE-Cy5.5, anti-CD25 PE, and anti-Foxp3 Alexa Fluor 488 (Human Treg Flow Kit; BioLegend) following the manufacturer's instructions. FCM experiments were performed and analyzed in Plateforme de Cytométrie IFR 100 Santé STIC, at Burgundy University. Data were acquired on a Beckman Coulter Epics-XL or a BD Bioscience LSRII cytometer and analyzed with FlowJo Version 5 software. Anti-CD3 (Neomarkers); anti-CD4 (Novocastra); anti-Foxp3 (clone 236A/E7; Abcam); anti-CD20, anti-CD79a, and anti-CD138 (Dako); and anti-κ and anti-λ light-chain (BD Biotech) antibodies were used for immunohistology.
Assessment of Th-cell polarization
Negative selection of CD4+ lymphocytes was performed using a CD4+ T-Cell Isolation Kit II (Miltenyi Biotec). FCM analyses showed a purity of at least 90% of CD4+ cells. CD4+ T lymphocytes (5 × 105 cells) were cultured in 24-well plates in 1 mL of RPMI 1640 (Bio Whittaker) with 10% of FBS (GIBCO-BRL) and stimulated with 0.1 μg/mL of phorbol 12-myristate 23-acetate (PMA) and 1 μg/mL of ionomycin (Sigma-Aldrich) for 8 hours in the presence of 1 μL/mL of brefeldin A (BD Golgi Plug; BD Biosciences) for the last 4 hours. CD4+ lymphocytes were isolated before cytokine staining because PMA stimulation triggers internalization and degradation of the CD4 receptor, which interferes with the identification of Th1 (CD4+IFN-γ+), Th2 (CD4+IL-4+), and Th17 (CD4+IL-17+) cells.29 The cells were harvested, fixed, and permeabilized (Fixation Permeabilization buffer; eBioscience) for intracellular cytokine staining using anti–IFN-γ APC, anti–IL-17 PE, and anti–IL-4 FITC antibodies (eBioscience).
RT-PCR
Total RNA was isolated from splenic CD4+ cells. IL-17A, IFN-γ, RORc, T-bet, GATA3, and Foxp3 transcripts were quantified by RT-PCR and normalized to the expression of L32. The following primer sequences were used: IL-17A: 5′-AGCCTGGAGGCCATAGTGAA-3′, 5′-CGGGGGAAG- TTCTTGTCCTC-3′; IFN-γ: 5′-CTGTCGCCAGCAGCTAAAAC-3′, 5′-ACTGGGATGCTCTTCGACCT-3′; RORc: 5′-TCTGGAGCTGGCCTTT- CATC-3′, 5′-CAGCTTTGCCAGGATGCTTT-3′; T-bet: 5′-CCCCTTGGTGTGGACTGAGA-3′, 5′-ACGCGCCTCCTCTTAGAGTC-3′; GATA3: 5′-GTCCTCCCTGAGCCACATCT-3′, 5′-GTGGTCCAAAGGACAGGCTG-3′; and Foxp3: 5′-CAGCTGCAGCTGCCCAC-3′, 5′-TGTCCTGGAG- GAGTGCCT-3′.
Statistical analysis
Nonparametric tests were used because normality of distribution or homoscedasticity were not verified. Kruskal-Wallis and Mann-Whitney tests were used to compare percentages and absolute count of cells between the different groups. The Wilcoxon test was used for paired values (Tregs and blood B cells before and after RTX). Results were considered statistically significant when P < .05. Analysis was performed with STATA Version 10 (Stata Corporation) and Prism (GraphPad) software.
Results
RTX therapy reduced the number of circulating B cells but did not affect the frequency of Tregs in the blood
The percentages of circulating Tregs and B cells were first assessed in 27 ITP patients at the time of diagnosis before therapy and in the 28 control individuals. The percentage and absolute count of circulating CD4+CD25HighFoxp3+ Tregs was comparable in controls and ITP patients (4.25 ± 1.1 vs 3.8 ± 1.56% of CD4+ lymphocytes, nonsignificant; Figure 1A; 41.9 ± 19.2 vs 34.6 ± 19.7 × 106 cells/L, nonsignificant). The level of circulating B cells was also similar in the 2 groups (10.7 ± 4.3 vs 10.6 ± 5.7% of total lymphocytes, nonsignificant; Figure 1A). RTX therapy resulted in a significant decrease in the percentage of circulating B cells (10.3% ± 6.9% to 0.07% ± 0.28% of total lymphocytes, P = .0016; Figure 1B) but did not modify the percentage of circulating Tregs (mean 4.5% ± 1.7% before vs 4.7% ± 2.5% of CD4+ lymphocytes after RTX, n = 8, nonsignificant; Figure 1B). RTX-mediated B-cell depletion did not lead to significant clinical improvement in 7 of 13 ITP patients. Because the immunomodulatory effects of RTX in secondary lymphoid organs in humans are unclear, spleens removed from ITP patients treated or not with RTX were further analyzed.
Circulating levels of Tregs and B cells. (A) CD4+CD25HighFoxp3+ Tregs were analyzed by FCM in controls (n = 26) and ITP patients (n = 20) at diagnosis. Dot plots of Treg levels are expressed as a percentage of CD4+ lymphocytes (left panel). Dot plots of circulating CD19+ B-cell levels observed in controls (n = 28) and ITP patients (n = 27) are expressed as a percentage of total circulating lymphocytes (right panel). The horizontal bars represent the mean values with standard deviations. (B) Circulating Treg levels expressed as a percentage of CD4+ lymphocytes were also measured before and after RTX in 8 patients (left panel). Circulating CD19+ B-cell levels assessed by FCM before and after RTX infusion (n = 13) are expressed as a percentage of total circulating lymphocytes (right panel). *P < .05; **P < .001; ***P < .0001; NS, nonsignificant.
Circulating levels of Tregs and B cells. (A) CD4+CD25HighFoxp3+ Tregs were analyzed by FCM in controls (n = 26) and ITP patients (n = 20) at diagnosis. Dot plots of Treg levels are expressed as a percentage of CD4+ lymphocytes (left panel). Dot plots of circulating CD19+ B-cell levels observed in controls (n = 28) and ITP patients (n = 27) are expressed as a percentage of total circulating lymphocytes (right panel). The horizontal bars represent the mean values with standard deviations. (B) Circulating Treg levels expressed as a percentage of CD4+ lymphocytes were also measured before and after RTX in 8 patients (left panel). Circulating CD19+ B-cell levels assessed by FCM before and after RTX infusion (n = 13) are expressed as a percentage of total circulating lymphocytes (right panel). *P < .05; **P < .001; ***P < .0001; NS, nonsignificant.
RTX therapy induced marked follicular atrophy and a significant reduction in the number of splenic B lymphocytes
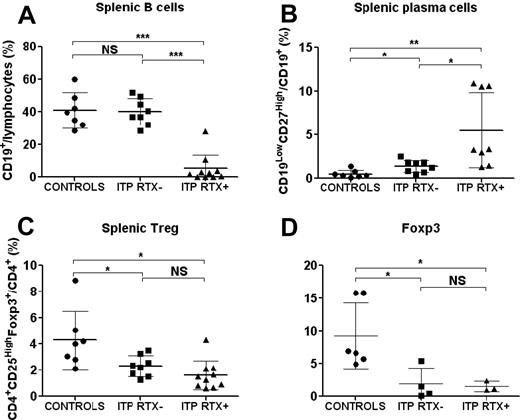
The quantification and distribution of different immune cell subsets were first assessed by immunohistology in the spleens of controls (n = 4), RTX-treated ITP (ITP RTX+; n = 4), and RTX-untreated ITP (ITP RTX−; n = 6; supplemental Figure 1 and supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Follicular hyperplasia consistent with secondary follicles with germinal centers was observed in RTX-untreated ITP patients. These secondary follicles accounted for 82.5% of the total follicles in ITP patients compared with 7.5% in controls (P = 0.013). RTX induced marked follicular atrophy associated with a reduction in the number of CD20- and CD79a-expressing cells (supplemental Figure 1 and supplemental Appendix). Splenic cells were also quantified by FCM in 7 controls and 18 ITP patients, among whom 10 had previously been treated with RTX. No significant difference was observed in the number of B cells, CD4 and CD8 lymphocytes, and natural killer cells in ITP compared with control spleens (data not shown). The data depicted in Figure 2A indicate that RTX therapy resulted in a significant decrease in the frequency of splenic B lymphocytes in RTX-treated ITP patients compared with control individuals (5.1% ± 8.7% vs 40.8% ± 10.7% of splenic lymphocytes; P = .0001) or to RTX-untreated ITP patients (5.1% ± 8.7% vs 40.1% ± 8.1% of splenic lymphocytes; P < .0001). Residual B cells accounted for < 1% of lymphocytes in 4 patients, ranged from 1%-5% in 4 patients, and represented > 10% of splenic lymphocytes in 2 patients. However, the number of residual B cells was correlated with the time between RTX infusion and splenectomy (linear regression, P = .013, R2 = 0.56; supplemental Figure 2 and supplemental Appendix), which is consistent with splenic B-cell regeneration after RTX therapy. A significantly greater number of plasma cells, defined as CD19LowCD27High cells (supplemental Figure 3 and supplemental Appendix), was observed in the spleens of RTX-untreated ITP patients than in control spleens (1.37% ± 0.7% vs 0.5% ± 0.4% of CD19+ cells, P = .017; Figure 2B). In ITP patients treated with RTX, the frequency of these plasma cells was even further increased (5.5% ± 4.3% vs 0.5% ± 0.4% of CD19+ cells, P = .002; Figure 2B). Plasma cells were localized by immunohistology using anti-kappa and anti-lambda light-chain intracellular staining. In controls and ITP patients, plasma cells were preferentially found in the red pulp around the vessels. In ITP patients, they were also detected around periarteriolar lymphoid sheaths and rarely in germinal centers of secondary follicles of RTX-untreated ITP patients (supplemental Figure 1 and supplemental Appendix). CD138 staining was negative. Circulating plasma cell levels measured before treatment in 9 controls and 8 ITP patients were not significantly different (data not shown). These results demonstrate that although RTX induced a nearly complete depletion of splenic B cells in most patients, these patients did not achieve remission, which might be explained by the high number of remaining plasma cells detected in the spleens of these ITP patients after RTX therapy. However, the involvement of cytotoxic T cells in platelet lysis could not be definitively excluded.
Splenic Treg, B-cell, and plasma-cell levels. After mechanical disruption of the spleen, Tregs were defined on FCM by the phenotype CD4+CD25HighFoxp3+, B cells as CD19+ lymphocytes, and plasma cells as CD19LowCD27High cells. The data are summarized in dot plots representing percentages of B cells (A), plasma cells (B), and Tregs (C) in controls (n = 7), ITP patients not treated with RTX (ITP RTX−; n = 8), and ITP patients previously treated with RTX (ITP RTX+; n = 10). (D) Foxp3 expression was measured by RT-PCR in sorted splenic CD4+ T cells in controls (n = 6), ITP RTX− (n = 4), and ITP RTX+ (n = 3). The horizontal bars represent the mean values with standard deviations. *P < .05; **P < .001; ***P < .0001; NS, nonsignificant.
Splenic Treg, B-cell, and plasma-cell levels. After mechanical disruption of the spleen, Tregs were defined on FCM by the phenotype CD4+CD25HighFoxp3+, B cells as CD19+ lymphocytes, and plasma cells as CD19LowCD27High cells. The data are summarized in dot plots representing percentages of B cells (A), plasma cells (B), and Tregs (C) in controls (n = 7), ITP patients not treated with RTX (ITP RTX−; n = 8), and ITP patients previously treated with RTX (ITP RTX+; n = 10). (D) Foxp3 expression was measured by RT-PCR in sorted splenic CD4+ T cells in controls (n = 6), ITP RTX− (n = 4), and ITP RTX+ (n = 3). The horizontal bars represent the mean values with standard deviations. *P < .05; **P < .001; ***P < .0001; NS, nonsignificant.
Numbers of splenic Tregs were reduced in ITP patients
FCM analysis indicated that the percentage of splenic CD4+CD25HighFoxp3+ Tregs was decreased in both RTX-untreated ITP patients (2.31% ± 0.8% vs 4.29% ± 2.23% of CD4+ T cells; P = .005) and RTX-treated ITP patients (1.6% ± 1.1% vs 4.29 ± 2.23% of CD4+ T cells; P = .01) compared with control individuals (Figure 2C). RT-PCR analysis of splenic cells confirmed that Foxp3 expression was significantly lower in RTX-untreated ITP patients (1.9 ± 2.4 vs 9.8 ± 5.5; P = .037) and RTX-treated ITP patients (1.5 ± 0.8 vs 9.8 ± 5.5; P = .036) than in controls (Figure 2D). Tregs identified as Foxp3+ cells by immunohistology were preferentially localized in periarteriolar lymphoid sheaths with an average frequency of 11 ± 7.1 in controls, 16.2 ± 5.4 in RTX-untreated ITP (nonsignificant), and 10.9 ± 5 cells in RTX-treated ITP patients (nonsignificant). In follicles, Tregs were sparser: 1.7 ± 1.8 cells in controls and 6.9 ± 3.6 cells in RTX-untreated ITP patients (supplemental Figure 1 and supplemental Appendix). No significant difference was observed between these groups. Because follicles were not detectable in RTX-treated ITP patients, Tregs could not be counted in this localization.
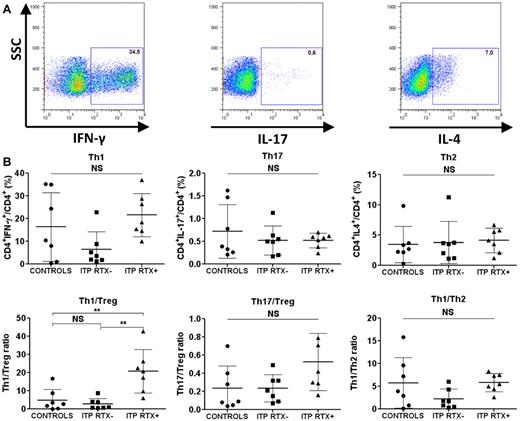
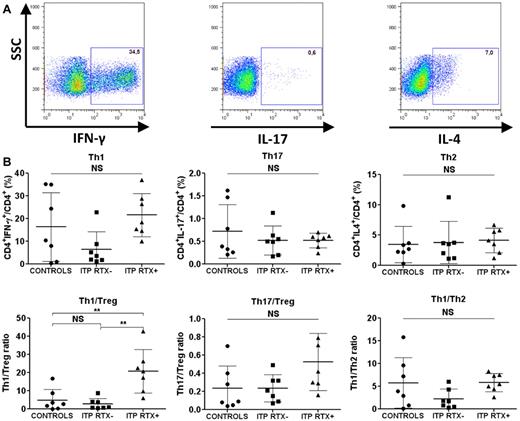
The Th1/Treg ratio was increased in the spleens of ITP patients refractory to RTX
Because autoimmunity primarily depends on the balance between pro- and anti-inflammatory cells, we focused on the splenic Th1/Treg and Th17/Treg ratios. Although no differences in splenic Th1, Th2, and Th17 levels or Th1/Th2 and Th17/Treg ratios were observed in RTX-treated and RTX-untreated ITP patients (Figure 3), a significant increase in the Th1/Treg ratio was detected in ITP patients who failed RTX therapy compared with controls (20.7% ± 12% vs 4.8% ± 6%; P = .004) and to ITP patients not treated with RTX (20.7% ± 12% vs 2.8% ± 3%; P = .001; Figure 3). RT-PCR also confirmed that the IFN-γ/Foxp3 mRNA ratio in RTX-treated ITP patients was significantly higher than that in controls (1113 ± 538 vs 80.7 ± 58.2; P = .028; Table 4). IFN-γ, T-bet, GATA3, RORc, and IL-17A mRNA expression were not significantly different between the 3 groups (Table 4). To assess whether the time between RTX infusion and splenectomy may have modified the investigated parameters, a correlative analysis was performed. No linear correlation was found between the duration between rituximab infusion and splenectomy and the following splenic parameters: Th1, Th1/Treg, Th2, Th17, Th17/Treg, and Treg percentages (data not shown).
Splenic T-cell responses. Sorted CD4+ T cells were stimulated with PMA and ionomycin for 8 hours. Brefeldin A was added for the last 4 hours. The cells were then fixed and permeabilized for IFN-γ, IL-17, and IL-4 intracellular staining. (A) Th1 cells were defined as CD4+IFN-γ+, Th17 cells as CD4+IL-17+, and Th2 cells as CD4+IL-4+. Dot plots are of one representative RTX-treated ITP patient. (B) Data are summarized in dot plots representing the different T-cell subsets (Th1, Th17, and Th2) in controls (n = 7), RTX-untreated ITP patients (ITP RTX−; n = 7), and RTX-treated patients (ITP RTX+; n = 7). The balance between immune response and immune tolerance was evaluated by assessing the Th1/Treg and Th17/Treg ratios. The Th1/Th2 ratio is also represented. The horizontal bars represent the mean values with standard deviations. *P < .05; **P < .001; ***P < .0001; NS, nonsignificant.
Splenic T-cell responses. Sorted CD4+ T cells were stimulated with PMA and ionomycin for 8 hours. Brefeldin A was added for the last 4 hours. The cells were then fixed and permeabilized for IFN-γ, IL-17, and IL-4 intracellular staining. (A) Th1 cells were defined as CD4+IFN-γ+, Th17 cells as CD4+IL-17+, and Th2 cells as CD4+IL-4+. Dot plots are of one representative RTX-treated ITP patient. (B) Data are summarized in dot plots representing the different T-cell subsets (Th1, Th17, and Th2) in controls (n = 7), RTX-untreated ITP patients (ITP RTX−; n = 7), and RTX-treated patients (ITP RTX+; n = 7). The balance between immune response and immune tolerance was evaluated by assessing the Th1/Treg and Th17/Treg ratios. The Th1/Th2 ratio is also represented. The horizontal bars represent the mean values with standard deviations. *P < .05; **P < .001; ***P < .0001; NS, nonsignificant.
Discussion
The spleen is of particular interest in ITP patients because it is where the immune response is triggered and regulated.25,30 In keeping with previous reports,31,32 the main histologic feature of the spleens of ITP patients enrolled in our study was the presence of numerous hyperplastic follicles with a predominance of secondary follicles, underlying the key role of B cells in ITP pathogenesis. Because of the involvement of B cells in ITP pathogenesis, RTX has been used successfully as a therapeutic option.21,33 However, the immunomodulatory effects of RTX in ITP have not been clearly established. The therapeutic benefit of RTX may partly result from its ability to promote the depletion of B lymphocytes responsible for the production of antiplatelet auto-antibodies detected in the blood of ITP patients. However, this mode of action should be tempered because the presence of auto-antibodies can still be observed in responder patients, probably because of the persistence of plasma cells, primary antibody-producing cells that do not express CD20.33 Consistent with this concept, it has been documented that the inefficacy of RTX therapy in systemic lupus erythematosus was correlated with the persistence of circulating CD19LowCD27High plasma cells.27 In addition, the impact of RTX on immune cells in secondary lymphoid organs has only been investigated in isolated cases in humans27 and in monkeys.34 We reasoned that even when circulating B cells are eliminated, resistance to RTX treatment may be explained by incomplete B-lymphocyte depletion or by the persistence of plasma cells in secondary lymphoid organs. To address this hypothesis, we investigated for the first time the effects of RTX on splenic cells in patients with ITP. Although, as a limitation of our study, only 18 spleens from ITP patients were analyzed, we obtained significant new information. We demonstrated that RTX dramatically reduced the number of splenic B lymphocytes. Examination of the spleens from nonresponder patients treated with RTX 6 months before splenectomy revealed complete B-cell depletion (CD19+ lymphocytes < 1% of splenic lymphocytes) in 40% of these patients. In our study, we also demonstrated that the amount of residual B cells was correlated with the time between RTX infusion and splenectomy. These results demonstrate that depletion of both circulating and splenic B cells, which was achieved in most of the patients treated with RTX, was not sufficient to lead to a clinical response. Interestingly, plasma cells were still detected in the spleens of patients treated with RTX. Because splenic cells do not express CD138, these plasma cells are likely to be short-lived plasma cells repopulating the spleen rather than persisting long-lived plasma cells. However, because antiplatelet antibodies and the presence of plasma cells in BM have not been assessed, a possible lack of efficacy of RTX on long-lived plasma cells in other sites than the spleen cannot be ruled out. Further studies are needed to clarify the involvement of these 2 different types of plasma cells in ITP pathogenesis, particularly as it relates to their respective role in the production of antiplatelet antibodies. However, because long-lived plasma cells are only localized in particular niches such as BM, secondary lymphoid organs, or inflamed tissues, they are particularly difficult to study in humans.35
It has been reported previously that in ITP patients, RTX corrects peripheral T-cell dysregulation such as antiapoptotic protein overexpression, increases in the Th1/Th2 and Tc1/Tc2 ratios, and quantitative or functional defects of Tregs.16,24 However, whether RTX may modulate immune cells in the spleens of ITP patients has not yet been investigated. We provide evidence for the first time that an increase in the splenic Th1/Treg ratio was observed in RTX-treated patients. Surprisingly, no significant Th1 polarization or imbalance between effector cells and inhibitory cells such as Th1/Treg or Th17/Treg ratios were observed in the spleens of ITP patients not treated by RTX. ITP is considered to be a Th1-mediated disease because of the increase in the Th1/Th2 ratio, which is determined by measuring cytokine RNA expression,11 serum cytokines,12 or intracellular cytokines.24 However, all of these results have been generated from peripheral blood samples, and such investigations have never been performed in the spleens of ITP patients. Because the spleens of ITP responder patients were not available, we were not able to assess whether the observed increase in the Th1/Treg ratio was because of a resistant phenotype to RTX or was the consequence of RTX therapy. However, this imbalance in the Th1/Treg ratio may also be associated with other platelet destruction mechanisms, such as the stimulation of cytotoxic T cells that have previously been involved in some ITP patients.36 Cytotoxic T-lymphocyte participation in platelet destruction, particularly in patients who failed RTX treatment, therefore requires further investigation.
The role of circulating Tregs in ITP pathogenesis, particularly their reduced functionality, has been documented.14,16,17 Interestingly, defective Treg function in ITP can be normalized after treatments to restore platelet numbers, such as RTX16 or thrombopoietic agents.14 However, because Tregs were identified based on different phenotypes (CD4+CD25High, CD4+Foxp3+, or CD4+CD25HighFoxp3+), conflicting results were seen in the different studies.14-19 Our data support 2 recent studies that reported similar frequencies of circulating Tregs in ITP patients and controls.14,17 In contrast, other studies described a decrease in Treg number in ITP patients when Tregs were defined as CD4+CD25High15,19 or CD4+Foxp3+16,18 cells.
Because circulating Treg frequency is not necessary correlated with that of Tregs localized at the inflammatory site, such as has been observed in rheumatoid arthritis,37 we investigated Treg frequency in one of the main organs involved in ITP pathogenesis, the spleen. Although ITP patients had the same number of circulating Tregs as control individuals, the percentage of Tregs was significantly reduced in the spleens of ITP patients. This is an important finding, because it is related to the role of these immunosuppressive cells in the pathogenesis of ITP insofar as the spleen plays a major role in the modulation of immune responses in ITP. Interestingly, the frequency of splenic Tregs was not increased in the spleens of nonresponder patients. Whether RTX therapy restores Treg numbers in responder patients remains an open question because the spleens of these patients could not be studied.
In summary, we demonstrated that in humans, RTX eliminates nearly all circulating and splenic B cells, but in some patients this was not sufficient to lead to clinical response. We also showed that plasma cells persisted in the spleens of RTX-treated patients. Importantly, patients who failed RTX therapy exhibited a marked imbalance in the Th1/Treg ratio. These results highlight a shift from a B cell–mediated to a Th1-mediated disease, which suggests the possible involvement of cytotoxic T lymphocytes in ITP pathogenesis, as was proposed in a previous study.36 Whether cytotoxic T cells play a direct role in RTX failure deserves further investigation. Our data also suggest that the use of drugs that modulate both the Th response and Tregs in RTX-refractory patients may be of particular interest.38,39
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Philip Bastable for his help in writing the manuscript and Bertrand Godeau and Marc Michel from the Centre de référence des cytopénies auto-immunes de l'adulte for their constructive remarks and comments during the preparation of the manuscript.
This work was supported by a grant from the Direction de la Recherche Clinique du CHU de Dijon and the Conseil Régional de Bourgogne 2004 and 2010 (to S.A. and B.B.), and National Institutes of Health grant R01 CA104926 (to E.K. and N.L.).
National Institutes of Health
Authorship
Contribution: S.A. and B.B. were the principal investigators; S.A., N.L., E.K., and B.B. designed the study and discussed the results; S.A., V.L., S.B., B.L., and B.B. recruited the patients; S.A., M.S., J.G., M.C., N.J., J.F., M.T., T.P., L.M., and M.M. performed the experiments; P.R. and N.C. performed the splenectomies; S.A.-G. and S.A. analyzed the results; S.A. and B.B. coordinated the research; and S.A., N.L., N.J., and B.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Bonnotte, CR Inserm 866, IFR 100, Faculté de Médecine, 7 Boulevard Jeanne d'Arc, 21079 Dijon, France; e-mail: bernard.bonnotte@u-bourgogne.fr.