Abstract
Previous studies have suggested that, in patients with AL amyloidosis treated with high-dose melphalan and autologous stem-cell transplantation (HDM/SCT), the greatest benefit is seen in those patients achieving a hematologic complete response (CR). We analyzed a series of 421 consecutive patients treated with HDM/SCT at a single referral center and compared outcomes for patients with and without CR. Treatment-related mortality was 11.4% overall (5.6% in the last 5 years). By intention-to-treat analysis, the CR rate was 34% and the median event-free survival (EFS) and overall survival (OS) were 2.6 and 6.3 years, respectively. Eighty-one patients died within the first year after HDM/SCT and were not evaluable for hematologic and organ response. Of 340 evaluable patients, 43% achieved CR and 78% of them experienced an organ response. For CR patients, median EFS and OS were 8.3 and 13.2 years, respectively. Among the 195 patients who did not obtain CR, 52% achieved an organ response, and their median EFS and OS were 2 and 5.9 years, respectively. Thus, treatment of selected AL patients with HDM/SCT resulted in a high organ response rate and long OS, even for those patients who did not achieve CR.
Introduction
Immunoglobulin light chain (AL) amyloidosis is the most common form of systemic amyloidosis, with an incidence of 5-12 persons per million per year.1 In AL amyloidosis, clonal bone marrow plasma cells produce monoclonal light chains that misfold and deposit in tissues and organs as amyloid fibrils, resulting in progressive system and organ failure, and ultimately in death. Untreated patients with this disease have a dismal outcome, with a median survival of 10-14 months from diagnosis.2 Moreover, fewer than 5% of patients survived for > 10 years before the introduction of high-dose melphalan and stem cell transplantation (HDM/SCT).3 Oral melphalan and prednisone (MP) modestly increases the median survival to 16-18 months and rarely induces hematologic complete responses (CRs) or reversal of organ dysfunction.2,4,5 The introduction of HDM/SCT in the 1990s appears to have markedly improved these results.6 Single and multicenter studies show CR rates of 16%-67%, organ responses in 25%-45% of patients, and a median overall survival (OS) of ∼ 5 years.7-16 A case-control study demonstrated the benefit of this procedure for patients younger than 70 years compared with nontransplant regimens, most of them alkylator-based oral chemotherapy.17 A major issue in HDM/SCT for AL amyloidosis is the potential for high treatment-related mortality (TRM) because of underlying organ dysfunction in this disease. Some early multicenter series reported TRM as high as 40%, but recent reports from experienced single centers have reported a TRM rate in the range of 10%-15% as a result of improved selection of patients and better peritransplantation management.17-19
In previous reports, we and others have shown that patients who achieve CR after HDM/SCT have a higher rate of clinical (organ) response as well as a longer OS.9,19-22 The aim of this study was to investigate the long-term outcome of patients who failed to achieve CR after HDM/SCT compared with those who did in terms of organ response, event-free survival (EFS), and OS.
Methods
Patients
A total of 421 consecutive patients diagnosed with AL amyloidosis and treated with HDM/SCT (100-200 mg/m2) at the Amyloid Treatment and Research Program at Boston Medical Center from July 1994 to December 2008 were included in this analysis. Data were collected prospectively during this time period. Patients with multiple myeloma (≥ 30% bone marrow plasma cells, plasmacytoma with a monoclonal protein in serum and/or urine, lytic bone lesions, and/or hypercalcemia, n = 16) or other B-cell lymphoproliferative disorders (n = 16) associated with AL amyloidosis, as well as one patient with inadequate follow-up after transplantation, were not included. We have reported outcome data on some of these patients previously.9,19 The current analysis includes additional patients and longer follow-up, and provides a detailed analysis of the subgroup of patients failing to achieve hematologic CR.
Treatment schedule
Data collection was approved by the Institutional Review Board of Boston University Medical Center, and written informed consent was obtained from each patient, in accordance with the Declaration of Helsinki. Patients were screened for treatment with HDM/SCT according to the inclusion criteria of specific clinical trials or institutional eligibility requirements.9 Peripheral blood stem cells were collected by leukapheresis after mobilization using granulocyte colony-stimulating factor as previously reported. The dose of intravenous melphalan ranged from 100-200 mg/m2. A reduced dose of 100 or 140 mg/m2 was administered to patients older than 65 years, with a left ventricular ejection fraction between 40% and 45%, stem cell collection of 2 to < 2.5 × 106 CD34+ cells/kg, or with poor performance status. Complete assessments of hematologic and organ responses were done at 6 and 12 months after SCT and annually thereafter at Boston Medical Center. More frequent testing to monitor hematologic parameters and organ function was carried out by the patients' physicians as clinically indicated.
A total of 231 patients (55%) received the full high dose of melphalan (200 mg/m2), whereas 190 (45%) received a modified dose. Thirty-nine patients (9.2%) received a second cycle of HDM/SCT after failing to achieve CR after the first transplantation. Of these, 9 patients underwent tandem transplantation with 2 cycles of modified HDM at 100 mg/m2 according to Southwest Oncology Group trial S0115. Four patients underwent transplantation with 2 cycles of 200 mg/m2. Twelve patients underwent transplantation with 200 mg/m2 followed by a second cycle of 140 mg/m2. Thirteen patients received a first SCT with 140 mg/m2 followed by a second SCT at the same dose. One patient received 140 mg/m2 in a first SCT followed by a second SCT at 200 mg/m2.
Evaluation of response
Hematologic and organ responses were evaluated according to the Consensus Opinion from the Xth International Symposium on Amyloid and Amyloidosis.23 For patients treated before the serum free-light chain (FLC) test (FreeLite, the Binding Site) was available in our institution (2003), CR was defined as absence of monoclonal gammopathy by immunofixation electrophoresis of serum and urine and normal bone marrow biopsy (ie, absence of κ- or λ-clonal plasma cells by immunohistochemistry). For those patients who were treated after the serum FLC test was available, a normal serum immunoglobulin FLC ratio and concentration were also required in addition to the previous criteria to define a CR. At the time this study was carried out, a consensus for definition of partial response was not established. Thus, patients who failed to achieve a CR were not subdivided into partial responders and nonresponders in this analysis, and were termed non-CR.
The time point chosen to assess the hematologic response was 1 year after SCT. A landmark analysis was performed, including in the analysis the 340 patients who survived at least 12 months after HDM/SCT. For those patients who received a second cycle of HDM/SCT because of persistence of plasma cell dyscrasia after the first procedure, response was assessed at 1 year after the second course.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics and to analyze the variables of the study. The actuarial Kaplan-Meier method was used to estimate progression-free survival (PFS), EFS, and OS, all of them measured from the infusion of stem cells (day 0).24 Survival measurements were compared using the log-rank test.25 Patients who did not present any event (as described in “EFS and OS”) or survived through the end of the study period were considered censored observations. At the time of this analysis, the median follow-up is 4 years (range, 0-15.6 years) for the entire cohort and 6.3 years (range, 1-15.6 years) for surviving patients. Cox regression was used to compare PFS, EFS, and OS distributions in various risk groups while adjusting for potential confounders. We used multiple logistic regression to compare CR rates in various risk groups while adjusting for potential confounders. The assumption of proportional hazards was assessed before attempting Cox modeling. A value of P ≤ .05 was considered statistically significant. All analyses were performed using SAS Version 9.2 (SAS Institute).
Results
Patients
Patient characteristics are summarized in Table 1. A total of 421 patients were included in the study; 169 (40%) were women. The median age was 56 years (range, 28-78 years). The performance status was ECOG 1 in most cases and the median number of organs involved was 2 (range, 1-6). Involvement of 1 organ was present in 125 patients (30%), with isolated renal involvement in 80% of them; 2 organs were involved in 115 (27%), and 3 or more organs in the remaining 181 (43%). Distribution of organ involvement was as follows: kidney, 84%; heart, 45%; liver, 27%; autonomic neuropathy, 26%; soft tissue, 22%; gastrointestinal, 20%; peripheral neuropathy, 10%; and lung, 4.5%. Heart was the primary involved organ in 71 patients (16.8%). A total of 340 patients (80.8%) had a λ-clonal plasma cell dyscrasia, and the median percentage of plasma cells in the bone marrow was 5% (range, < 5%-25%). Sixty-eight patients (16%) had serum creatinine ≥ 2 mg/dL and 25 (5.9%) underwent SCT while on dialysis. The median time from the diagnosis of AL to the SCT was 5.2 months (range, 1 month to 12 years). A total of 102 patients (24.2%) had received treatment before HDM/SCT, consisting of melphalan plus prednisone in 74 patients, dexamethasone in 12, melphalan plus dexamethasone in 5, thalidomide with or without dexamethasone in 4, lenalidomide plus dexamethasone in 3, bortezomib in 2, and combination chemotherapy with vincristine, adriamycin, and dexamethasone in 2 patients.
Hematologic response
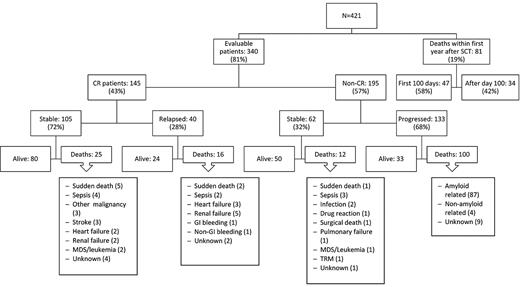
A total of 340 of the 421 patients (81%) were followed for at least 1 year to assess their hematologic response status (see flow chart in Figure 1). Eighty-one patients (19%) did not survive for 1 year to be evaluated for hematologic or organ response. A total of 145 (34% of all treated patients and 43% of the evaluable patients) achieved CR. Data on bone marrow plasma cell involvement were available in 406 of the 421 patients. Approximately half (197, 48%) had 10% to 25% bone marrow plasma cells by immunohistochemical staining of the biopsy specimen, whereas 209 had < 10% bone marrow plasma cells. The CR rate was 33% versus 36% in these groups, respectively, which was not statistically different (P = .48). After a median follow-up of 6.9 years (range, 1-15.6 years) for this “CR” population, hematologic relapses occurred in 40 patients (28%) and the estimated median time to relapse for the 145 patients in CR was 12.7 years (95% CI, 8.3 years to not attained). Twenty-six patients received second-line treatment because of evidence of clinical disease progression, which consisted of oral melphalan-based chemotherapy in 3 patients, regimens containing novel agents in 14 patients (3 thalidomide, 2 bortezomib, 9 lenalidomide), dexamethasone alone in 1 patient, and second SCT in 8 patients (7 autologous and 1 allogeneic).
Flow chart showing the outcome of 421 consecutive patients with AL amyloidosis treated with HDM-SCT.
Flow chart showing the outcome of 421 consecutive patients with AL amyloidosis treated with HDM-SCT.
A total of 66% of all treated patients (276 of 421) and 57% of the evaluable patients (195 of 340) did not achieve CR. Their characteristics are summarized in Table 1. Regarding the 195 patients who survived at least for 1 year and did not achieve CR, the median number of organ systems involved was 2, with 31% of patients having > 2 organs involved. At the time of this analysis, 68% (133 of 195) of the non-CR patients have had evidence of disease progression, with worsening organ function. The median time to progression was 2.7 years (95% CI, 2.0-3.2 years). Fifty-one percent of these patients (68 of 133) have received additional therapy with melphalan (4), thalidomide (21), bortezomib (8), lenalidomide (24), dexamethasone (8), a second autologous SCT (2), or a reduced-intensity allogeneic SCT (1). Two patients refused additional treatment after progression. Fifty (26%) of the non-CR patients remain alive and clinically stable, although 12 of this group have received further treatment because of persistence of clonal disease without evidence of clinical progression (thalidomide-based in 2, bortezomib-based in 1, lenalidomide-based in 8, and HDM/SCT in 1).
In univariate analysis, full-dose melphalan (P < .0001), absence of cardiac involvement (P = .005), normal FLC ratio (P = .04), κ-light chain isotype (P = .002), and alkaline phosphatase value > 200 mg/dL (P = .02) were significant predictors of CR. A multivariate analysis, including age, sex, melphalan dose, number of organs involved, cardiac involvement, light chain isotype, serum creatinine, and alkaline phosphatase values, showed that melphalan dose of 200 mg/m2 (P = .001), κ-light chain clonal disease (P = .007), absence of heart involvement (P = .015), and serum alkaline phosphatase more than 200 U/L (P = .037) were significant predictors for CR (Table 2). As noted in “Treatment schedule,” full-dose melphalan was generally administered to patients who were younger and had superior performance status and cardiac function. Table 3 shows the impact of full dose of melphalan compared with the intermediate doses. As reported previously,9 κ-light chain isotype also predicts for CR, but there was no difference in relapse/event rate or time to relapse/event based on light chain (data not shown). On the other hand, administration of treatment before the HDM/SCT did not significantly influence the probability of CR in our series. The hematologic CR rate was 41% for the 102 patients who received treatment before HDM/SCT, whereas it was 32% for the 319 patients without previous therapy (P = .12).
The multivariate model did not include serum FLC because it was not measured in all patients included in the study. However, we investigated the potential predictive value of pretransplantation involved FLC in 247 patients for whom this value was available. Patients achieving CR had a trend toward a lower pretransplantation involved FLC (P = .06). B type natriuretic peptide (BNP) measurement was available in 57 patients with cardiac involvement. BNP did not have predictive value for either hematologic CR (P = .6) or for organ response (P = .10) in this group of patients.
Organ response
Clinical (organ) response was assessed according to published consensus criteria.23 Any involved organ system with a potential of improvement was evaluated at 6 months, 12 months, and annually thereafter. Of the 145 patients who achieved CR after HDM/SCT, 114 (78.6%) had improvement in at least 1 involved organ. The organ response rate for those patients who did not achieve CR was 39.1% for all treated patients and 53.3% for those who survived at least 1 year after HDM/SCT. This lower rate of clinical response was significantly different from that of the CR patients (P < .0001).
We investigated the influence of pretransplantation involved FLC in the achievement of organ response in 247 patients. We found that lower baseline levels of the involved FLC predicted organ response after HDM/SCT (median of 93.3 mg/L in organ responders vs 133 mg/L in nonresponders, P = .01), although this predictive value was not observed for the subgroup of evaluable patients who did not achieve a CR (98.1 vs 104 mg/L, P = .62).
EFS and OS
To assess the outcome of the group of patients who survived for at least 1 year after HDM/SCT and did not achieve CR (“non-CR” population), EFS was determined. Events that were considered were hematologic or organ disease progression, initiation of additional therapy, or death. Median EFS in this non-CR population was 2.0 years (95% CI, 1.6-2.7 years). Number of organs involved (P = .03) and abnormal FLC ratio (P = .003) at baseline were the only predictors of EFS. No other baseline characteristics of the non-CR patients (age, sex, light chain isotype, presence of cardiac involvement, dose of melphalan, renal function, alkaline phosphatase, or involved FLC) predicted EFS.
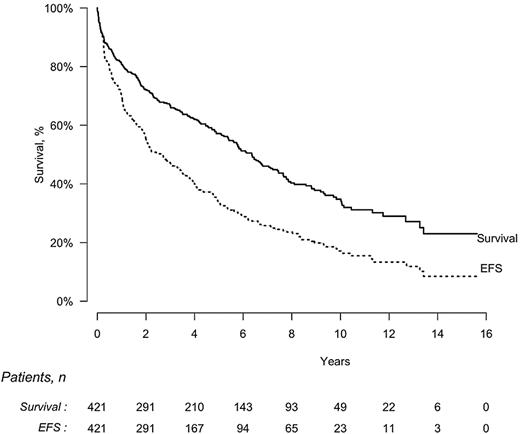
The median EFS of all 421 patients who received intravenous melphalan was 2.6 years (95% CI, 2.0-3.4 years), and the median OS was 6.3 years (95% CI, 5.4-7.4 years; Figure 2). On an intention-to-treat basis, median EFS was significantly longer for patients who achieved CR compared with those who did not (8.3 vs 1.0 years, P < .0001) as was OS (median of 13.2 vs 3.2 years, P < .0001). EFS was also significantly longer for patients who received the full dose of melphalan compared with a lower dose (3.4 vs 1.8 years, P = .0006) as was OS (8.4 vs 3.8 years, P < .0001; Table 3). OS was significantly longer for patients without cardiac involvement compared with those with cardiac disease (7.6 vs 3.4 years, P < .0001). On the contrary, OS was not influenced by administration of treatment before the HDM/SCT (5.6 vs 6.4 years in patients with and without previous treatment, respectively, P = .28).
EFS and OS by intention-to-treat analysis in 421 patients with AL amyloidosis treated with HDM/SCT. Median EFS, 2.6 years (95% CI, 2.0-3.4 years); median OS, 6.3 years (95% CI, 5.4-7.4 years).
EFS and OS by intention-to-treat analysis in 421 patients with AL amyloidosis treated with HDM/SCT. Median EFS, 2.6 years (95% CI, 2.0-3.4 years); median OS, 6.3 years (95% CI, 5.4-7.4 years).
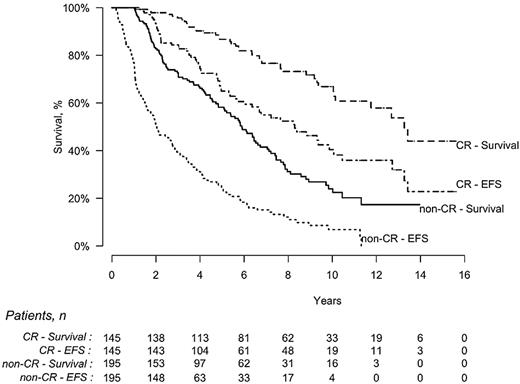
Among the 340 patients evaluable for response, EFS was statistically longer for patients achieving CR compared with those who did not (8.3 vs 2.0 years, P < .0001), and OS was also significantly longer (13.2 vs 5.9 years, P < .0001; Figure 3). The estimated probability of survival for patients in CR was 86% (95% CI, 79%-91%) at 5 years and 67% (95% CI, 57%-76%) at 10 years, whereas it was 58% (95% CI, 50%-65%) and 24% (95% CI, 16%-32%), respectively, for those who did not achieve CR.
EFS and OS of patients who achieved a hematologic CR (n = 145) after HDM/SCT compared with those who did not (non-CR, n = 195). EFS was significantly longer for CR patients (8.3 vs 2 years, P < .0001), as was OS (13.2 vs 5.9 years, P < .0001).
EFS and OS of patients who achieved a hematologic CR (n = 145) after HDM/SCT compared with those who did not (non-CR, n = 195). EFS was significantly longer for CR patients (8.3 vs 2 years, P < .0001), as was OS (13.2 vs 5.9 years, P < .0001).
In univariate analysis, female sex (P = .007), age < 65 years (P = .006), full-dose melphalan (P < .0001), < 3 involved organs (P < .0001), absence of cardiac involvement (P < .0001), low BNP levels (P = .029), normal FLC ratio (P < .001), and low pretransplantation involved FLC value (P < .0001) were significant predictors for survival. The multivariate analysis, including age, sex, dose of melphalan, light chain isotype, number of organs involved, cardiac involvement, serum creatinine, and alkaline phosphatase values, showed that melphalan dose of 200 mg/m2 (P = .001), involvement of < 3 organs (P = .0001), and female sex (P = .03) predicted better survival (Table 4). Again, this analysis did not include serum FLC, as it was not available in all patients included in the study.
Mortality
At the time of this analysis, 16 years after HDM/SCT program for AL was begun, 234 (55.5%) of the 421 patients have died. Eighty-one (19.2%) of them died within the first year after HDM/SCT; thus, they were considered nonevaluable in terms of assessment of hematologic and organ response. Among these 81 nonevaluable patients, causes of death were treatment-related in 47 (deaths within first 100 days after HDM/SCT) and disease-related in 34 (heart failure in 9, sudden death in 9, sepsis in 5, renal failure in 4, malnutrition in 2, unknown in 2, and pulmonary failure, nongastrointestinal bleeding, and hepatic failure one case each). An additional patient died within first 100 days after a second HDM/SCT. Thus, TRM in the overall series was 11.4%. Of note, the TRM has decreased to 5.6% for the 124 patients who received HDM/SCT in the last 5 years, despite the fact that the patients' clinical characteristics were similar in the 2 time periods (Table 5). Among patients who underwent HDM/SCT while on dialysis, the TRM within the first 100 days was 8% (2 of the25 patients). Interestingly, although not statistically significant, the TRM was lower in patients receiving the full dose of melphalan in our series (Table 3). In patients with cardiac involvement, BNP levels were predictive of mortality at 100 days (P = .01) and 1 year (P = .009).
Of the 340 patients who survived for at least 1 year, 153 patients have died: 41 of 145 (28%) who achieved CR and 112 of 195 (57%) who did not. Twenty-five patients who achieved CR died without evidence of hematologic relapse. Their causes of death included sudden death (5 patients), sepsis (4), other malignancy (3; bladder, lung, and metastatic adenocarcinoma of unknown origin one case each), stroke (3), heart failure (2), renal failure (2), therapy-related myelodysplastic syndrome/acute myeloid leukemia (2), and unknown (4). Among the 112 patients who did not achieve CR and died beyond the first year after SCT, 12 had no evidence of disease progression. Their causes of death included infection in 5 patients and sudden death, pulmonary failure, leukemia, drug reaction, post-surgical complications, transplantation-related mortality (after a second SCT), and unknown one case each.
A total of 104 of the 145 patients who achieved CR after HDM/SCT are still alive (71.7%) compared with 83 of the 195 (42.5%) patients who did not achieve CR (P < .0001).
Discussion
This study compares the benefits of HDM/SCT for patients with AL amyloidosis who achieve hematologic CR after transplantation with those who do not. Indeed, this series provides the longest outcome data on AL patients treated with HDM/SCT, including OS, EFS, and long-term mortality. Our results demonstrate that, with careful patient selection and experienced management, low rates of TRM can be achieved: 11.4% for all patients over 15 years and 5.6% in the last 5 years. This decrease in TRM has been also observed by other groups.26 Our selection criteria do not exclude patients based on age (17.3% patients were ≥ 65 years old), time from diagnosis to SCT (median of 5.2 months), number of involved organs (3 or more in 43% of patients), cardiac involvement (45% of patients), or renal failure (creatinine ≥ 2 mg/dL and end-stage renal disease in 16% and 5.9% of patients, respectively). Nonetheless, as for other diseases, AL amyloidosis patients who are eligible for HDM/SCT are healthier at baseline than those who are not, and eligibility for HDM/SCT is probably a favorable prognostic factor for survival of patients with AL amyloidosis, as it is for patients with other hematologic diseases.27
It is well established that the achievement of CR in the setting of HDM/SCT for AL amyloidosis is associated with higher organ response rate9 and longer survival.9,19-22 In the present study, the CR rate was 43% (145 of 340) in a landmark analysis of the 340 evaluable patients who survived for at least 1 year after transplantation and 34% of all patients undergoing SCT. Interestingly, 80 of these 145 patients are still alive and have not relapsed after a median follow-up of 6.9 years (range, 1-15.6 years). This group of patients represents 19% of the entire cohort of 421 patients, 52 of them surviving in continued CR for more than 5 years. Longer follow-up will reveal whether or not these patients are “operationally” cured of their disease, as it has been reported for a fraction of patients with multiple myeloma.28
Despite the importance of attaining CR, achievement of partial response does not mean treatment failure. Other authors have shown that patients who achieve partial response have an improved outcome with longer survival compared with patients with nonresponsive disease.22,29 In the current series, the group of patients who were evaluable at 1 year after HDM/SCT and did not achieve CR (57%) had a median OS of 5.9 years. In addition, median time from SCT to progression, additional treatment, or death of any cause in our non-CR population was 2.0 years. Interestingly, 50 (26%) of these patients are still alive and remain stable after a median follow-up of 3.8 years (range, 1-11.8 years). In our series, number of involved organs and abnormal FLC ratio at baseline were the only predictors of EFS among the clinical characteristics of the non-CR patients analyzed. Further possible predictive factors that might have relevance in the management of these patients should be investigated.
Importantly, in addition to these data on the duration of hematologic responses, we also observed organ responses in approximately half of the patients who did not achieve CR after HDM/SCT (39% when including the 81 patients who did not survive at least for 1 year), confirming the capacity for recovery of organ function when the underlying amyloidogenic light chain clone is significantly decreased after HDM/SCT. Good partial responses may shift the balance of amyloid deposition versus resorption in some patients. Based on these results, it is appropriate to withhold additional treatment for patients who do not achieve CR after SCT until there is clear evidence of progressive disease. Post-transplantation consolidation or maintenance therapy should only be administered in the context of clinical trials, as its benefit is as yet unproven.
Regarding the impact of prognostic factors on the achievement of CR, our results show the positive influence of κ-light chain isotype, full dose of melphalan, absence of cardiac involvement, and high alkaline phosphatase. With respect to survival, our results demonstrate the positive effect of having only one or 2 vital organs involved, full melphalan dose, and female sex. It is surprising that cardiac involvement did not retain its statistical significance in the multivariate analysis. This might reflect the exclusion of patients with severe cardiac involvement from HDM/SCT, reducing its impact as an independent factor in the model.
Recently, the combination of oral melphalan and pulse dexamethasone in selected patients has resulted in a hematologic response rate of 52%-67%, organ responses in 39%-48% of patients, and a median OS of ∼ 5 years.30,31 This regimen was compared with HDM/SCT, and outcomes and survival were similar.30 However, the results of this multicenter study raised a number of concerns, and the relative benefit of these 2 approaches remains uncertain. Our results highlight the importance of rigorous patient selection and multidisciplinary care in centers familiar with management of these complex patients, and support the use of HDM/SCT in patients who meet eligibility requirement and are eligible for full-dose melphalan chemotherapy. However, the benefits for treatment at reduced melphalan dosing are less clear. In this series, we found that treatment with a lower dose of melphalan resulted in a lower CR rate of 24.2%, median EFS of 1.8 years, and median OS of 3.8 years. Hematologic responses with novel agents, particularly bortezomib, can exceed this32,33 ; however, the organ response rate and the duration of hematologic responses seem to be lower than with HDM/SCT. Longer follow-up and randomized trials will be necessary to determine the optimal first-line therapy.
In conclusion, we report that treatment of patients with AL amyloidosis with HDM/SCT results in durable hematologic responses, a high organ response rate, and prolonged OS, even in those who do not achieve CR. These results can be obtained with a low TRM in patients who meet strict eligibility requirements for transplantation after an evaluation by a multidisciplinary team in an experienced center. There is no doubt that novel agents will be incorporated into both transplantation and nontransplantation regimens for AL amyloidosis.34 In the transplantation setting, clinical trials will investigate their role in induction, as part of conditioning, or for consolidation/maintenance after transplantation. Although more work is ahead to answer these questions, the results of this study reiterate the safety and potential for durable hematologic and organ responses to HDM/SCT in AL amyloidosis, even for patients who do not achieve CR.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anna Badiee for her assistance in the design of the database.
This work was supported in part by the National Institutes of Health (grant P01 HL68705), the Amyloid Research Fund at Boston University School of Medicine, and Instituto de Salud Carlos III (grant CM07/00108).
National Institutes of Health
Authorship
Contribution: M.T.C., V.S., D.C.S., and M.S. designed the study; M.T.C. analyzed the data and wrote the manuscript; V.S., D.C.S., J.B., and M.S. critically reviewed the manuscript; V.S., D.C.S., K.Q., J.L.B., L.M.D., A.S., F.R., H.M.-E., N.T.A., J.M.S., K.T.F., and M.S. took care of the patients; G.D. performed statistical analysis; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: J.B. has received honoraria for lectures, advisory boards, and grant research from Jansen-Cilag and Celgene. M.T.C. has received honoraria for lectures from Jansen-Cilag and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Martha Skinner, Amyloid Treatment and Research Program, K503, Boston University School of Medicine, 715 Albany St, Boston, MA 02118; e-mail: mskinner@bu.edu.