Abstract
Bruton tyrosine kinase (Btk) is essential for B cell development and function and also appears to be important for myeloid cells. The bone marrow of Btk-deficient mice shows enhanced granulopoiesis compared with that of wild-type mice. In purified granulocyte-monocyte-progenitors (GMP) from Btk-deficient mice, the development of granulocytes is favored at the expense of monocytes. However, Btk-deficient neutrophils are impaired in maturation and function. Using bone marrow chimeras, we show that this defect is cell-intrinsic to neutrophils. In GMP and neutrophils, Btk plays a role in GM-CSF– and Toll-like receptor–induced differentiation. Molecular analyses revealed that expression of the lineage-determining transcription factors C/EBPα, C/EBPβ, and PU.1, depends on Btk. In addition, expression of several granule proteins, including myeloperoxidase, neutrophilic granule protein, gelatinase and neutrophil elastase, is Btk-dependent. In the Arthus reaction, an acute inflammatory response, neutrophil migration into tissues, edema formation, and hemorrhage are significantly reduced in Btk-deficient animals. Together, our findings implicate Btk as an important regulator of neutrophilic granulocyte maturation and function in vivo.
Introduction
Bruton tyrosine kinase (BTK) belongs to the Tec family of non-receptor tyrosine kinases. Mutations in the BTK gene cause X-linked agammaglobulinemia (XLA) in humans. XLA is a severe, inherited immunodeficiency disease that is primarily considered to be a disorder of B cell development. BTK-deficient B cells are arrested at the pre-B cell stage in the bone marrow, which finally leads to an almost complete loss of peripheral B cells and, consequently, to a loss of primary and secondary immunoglobulins.1 Therefore, after the decrease in maternal immunoglobulins, affected males are highly susceptible to bacterial infections.2,3
BTK expression is not restricted to B cells; it is also found in hematopoietic stem cells (HSC), multipotent progenitors, and in many other hematopoietic cells, including myeloid cells, erythrocytes, and platelets.1 Over the past years, it became evident that BTK-deficiency, in addition to its dramatic effects on B-cell differentiation and signaling, also affects the myeloid compartment.4-10 However, the physiological consequences and the impact of the potentially impaired myeloid cell function for the overall immunodeficiency of XLA is still a matter of dispute.11-13
One of the documented clinical features of XLA is neutropenia.14-18 Two recent studies analyzing large cohorts of XLA patients showed that 11% to 26% experienced episodes of profoundly reduced numbers of neutrophils circulating in the peripheral blood.3,19 Although neutropenia in XLA patients was not associated with a specific BTK gene mutation, in most cases the mutation abolished BTK protein expression.19 The decrease in neutrophil numbers in the peripheral blood has been attributed to toxic effects of invading pathogens. However, in bone marrow samples from XLA patients, Kozlowski and Evans15 found evidence for a maturation arrest of neutrophils at the myelocyte/promyelocyte stage in 3 of 6 patients. It remains unknown whether this impaired myeloid differentiation results from systemic alterations or from cell-intrinsic defects in myeloid progenitors in XLA patients.
To shed light on the enigmatic role of Btk in myelopoiesis, we purified and analyzed murine Btk-deficient myeloid progenitors to assess their developmental potential and their molecular defects. Hematopoietic progenitors of the myeloid lineage can be subdivided into 3 main progenitor populations: the common myeloid progenitors (CMP) that differentiate either into megakaryocyte/erythrocyte progenitors (MEP) or into granulocyte-monocyte-progenitors (GMP).20,21 GMP differentiate mainly into monocytes/macrophages, neutrophils, and dendritic cells, and, to a minor extent, mast cells as well as eosinophils and basophils.22
Lineage commitment and subsequent differentiation are regulated by growth and differentiation factors, and by the expression level of transcription factors. For myeloid lineage diversification, important transcription factors include GATA-1, C/EBPα, and PU.1.22 Specifically, the ratio of C/EBPα to PU.1 expression levels determines the neutrophilic granulocyte versus monocyte/macrophage lineage decision. Moreover, C/EBPα directs the development of GMP by inhibiting lymphoid-specific transcription factors like Pax5.23
Here, we demonstrate a crucial role for Btk in GM-CSF- and Toll-like receptor (TLR)–driven development of purified GMP. In GMP, Btk is important for the expression of lineage determining transcription factors like C/EBPα and PU.1, and for the expression level of C/EBPβ, mainly important during late24 and “emergency” granulopoiesis.25 Btk-deficient neutrophils are impaired in expression of several granule proteins, and remain functional hampered in the Arthus reaction. Collectively, we provide evidence for a cell-intrinsic role of Btk in development and function of neutrophilic granulocytes.
Methods
Mice
Btk-deficient and wild-type littermates (both in the C57BL/6 background), CBA/J, and CBA/CaHN/Xid/J mice 8 to 18 weeks of age were purchased from The Jackson Laboratory. CD45.1+ mice were purchased from Charles River Laboratories. For the generation of chimeric mice, 5 × 106 bone marrow cells were injected intravenously into 8-week-old CD45.1+ animals that had been lethally irradiated with 1100 rad. All animal experiments were performed with the approval of the Ulm University institutional animal ethics committee.
BrdU-incorporation
The APC-BrdU-Flow-Kit (BD Pharmingen) was used. After a single intraperitoneal injection (1 mg/6 g of mouse weight) BrdU was given at 1 mg/mL with drinking water for 4 days.
Flow cytometry
The following antibodies were purchased from eBioscience: anti-CD34 (RAM34), anti–MHC class II (M5/114.15.2), anti-CD8a (53-6.7), anti-CD45R/B220 (RA3-6B2), anti-CD11b (M1/70), anti–IL-7Ra (A7R34), anti-Sca1 (D7), anti-CD117 (ACK2), anti-F4/80 (BM8), anti-CD16/32 (93), anti–Gr-1 (RB6-8C5), anti-CD45.1 (A20), and anti-CD45.2 (104); from BD: anti-CD11b (M1/70), anti-CXCR4 (2B11), anti-CD45R/B220 (RA3-6B2), and anti-CD11c (HL3); from BioLegend: anti-CD48 (HM48-1), anti-CD150 (TCF15-12F12.2), and anti-CD45 (30-F11); and from Miltenyi Biotec: anti-CD3e (145-2C11), anti-CD19 (6D5), anti–Gr-1 (RB6-8C5), and anti-Ter119 (Ter-119). FACS was performed on a FACSCanto II, and the data were analyzed with FACSDiva 6.2 software (BD).
Blood analysis
Blood was collected from the tail vein and analyzed using an Animal Blood Counter (Scil animal care company GmbH) and by flow cytometry.
Isolation of hematopoietic progenitor cells
Bone marrow cells were enriched for lineage-negative cells using antibodies against CD11b, CD19, Terr119, and rat-IgG-Dynabeads (Invitrogen). For sorting of GMP, lineage-depleted cells were blocked with mouse IgG (Jackson ImmunoResearch Laboratories) and stained with lineage markers (anti-CD3ϵ, anti-CD4, anti-CD8α, anti-CD45R, anti–Gr-1, anti-CD19, anti-CD11b, and anti-TER119) as well as anti–IL-7rα, anti-Kit, anti-Sca1, anti-FcγRII/III, and anti-CD34. Cells were sorted by FACSAria (BD).
In vitro colony-forming assays
Cell sorter-purified progenitors were placed in MethoCult M3231 supplemented with 50 ng/mL SCF, 10 ng/mL IL-3 and 25 ng/mL GM-CSF (StemCell Technologies) or supplemented with 50 ng/mL SCF, 10 ng/mL IL-3, 100 ng/mL Flit-3-ligand (R&D Systems), and either 10 μg/mL LPS (InvivoGen) or 1 μg/mL Pam3CSK4 (InvivoGen). At day 6, colonies were counted. To avoid massive cell death, colonies were analyzed phenotypically after 8 days of culture.26
Cytospins of CFU
Cytospins (Cytospin3 Cytocentrifuge, Thermo Shandon) were stained by Pappenheim and analyzed in a blinded manner using the Leica Microscope DM IRB or the Olympus scan screening station.
Reverse-passive Arthus reaction
The Arthus reaction was performed as described previously.27 Ear thickness was measured with a spring-loaded Oditest caliper (Kroeplin). Pictures were taken using a stereomicroscope with AxioCam MRc (Carl Zeiss) and AxioVision software (Version 4.7.1). The extent of hemorrhage was quantified by evaluating the number and size of hemorrhages per ear as described previously.28
Electron microscopy
Immunofluorescence staining
Ears were embedded in Tissue-Tek O.C.T. compound (Sakura), frozen in a dry-ice/isopentane bath, and stored at −80°C. Cryostat sections of 4 μm were prepared, air dried, and fixed in acetone. Immunofluorescence staining was performed using anti–Gr-1–FITC (BD), biotinylated anti–CD31-IgG (BD), and Streptavidin-PE-labeled IgG (eBioscience), or using anti–mouse-CD45.2 (eBioscience), anti–rat-Kit (eBioscience), and goat-anti–mouse-AlexaFluor488, donkey-anti–rat-AlexaFluor594 (Invitrogen), and DAPI (0.1 μg/mL; Roche). Slides were analyzed with a fluorescence microscope (Axiovert 200M; Carl Zeiss) equipped with AxioCam MR3 (Carl Zeiss) and AxioVision software (Version 2.2.5).
Toluidin staining
Toluidin staining is described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Isolation of murine bone marrow PMN
Bone marrow cells were loaded on top of a discontinuous Percoll gradient (50%/55%/62%/81%) and centrifuged at 1600xg. PMN were harvested from the 62%/81% interface, loaded onto Histopaque 1119 (Sigma-Aldrich), and centrifuged at 1600g. PMN viability was > 95%, purity was > 70%. Where indicated, bone marrow cells were loaded onto Histopaque 1119 (Sigma-Aldrich) and centrifuged at 1600g for the depletion of erythrocytes, or neutrophils were isolated from the bone marrow using microbeads coupled with anti-CD11b antibodies (Miltenyi).
Analysis of neutrophil elastase and gelatinase activity
The EnzCheck Elastase Assay Kit and Gelatinase Assay Kit (E-12056, E-12055; Invitrogen) were used according the manufacturer's instructions to analyze enzyme release into the supernatant upon immune-complex-induced degranulation of neutrophils, as described elsewhere.27
Immunoblotting
RIPA-buffer prepared protein lysates were separated on 12.5% SDS-polyacrylamide gels. The following antibodies were purchased from Cell Signaling: anti-Akt, anti–phospho-Akt (Thr308), anti–phospho-GSK-3β (Ser9), anti–phospho-PI3K p85 (Tyr458), anti-Stat3, and anti–phospho-Stat3 (Tyr705); from BD: anti-Btk and anti-Btk (pY551)/Itk (pY551); and from Santa Cruz Biotechnology: anti-C/EBPα (14AA), anti-ERK2 (C-14).
RT-PCR
Real-time PCR was performed and analyzed as described previously.31 PCR primer sequences are presented in supplemental Table 1. Primers were synthesized by the company biomers.net.
Results
Significant alterations in all myeloid cell populations in Btk-mutant mice
Mutations in the murine Btk gene lead to X-linked immunodeficiency (Xid) that resembles XLA in humans but is less severe.32-35 Two different mouse models are available to study Btk function: the Xid-mice, in which a spontaneous mutation within the Btk gene (R28C) leads to the expression of a functional inactive Btk protein,34,35 and the Btk-deficient mice, in which Btk expression is abolished via gene targeting.32,33 First, bone marrow, blood, and spleens of wild-type and Btk-deficient mice were analyzed for the cellularity as well as for the frequency of myeloid cell types.
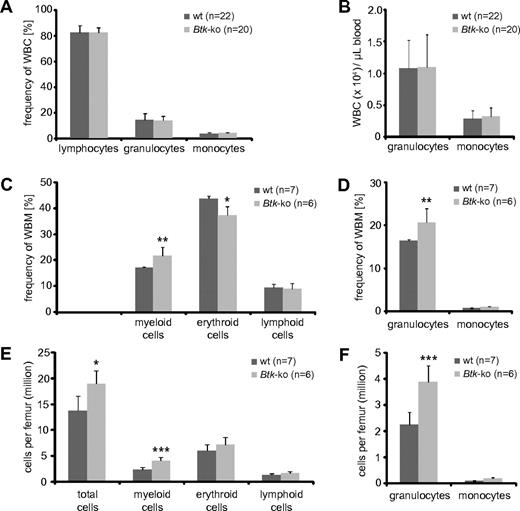
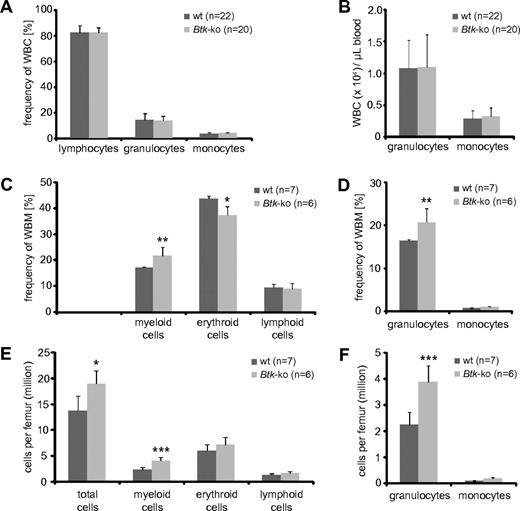
We observed a strong decrease in peripheral B cells that lead to a reduction of splenic cellularity, which has been described previously32,33 (supplemental Figure 1A-B). In addition, we observed a significant reduction of myeloid cells, mainly granulocytes and macrophages (supplemental Figure 1C-F). However, in the blood of Btk-deficient mice, no changes in the frequency or number of myeloid cells could be detected (Figure 1A-B). Similarly, analyses of Xid-mice revealed no significant changes in granulocytes and monocytes numbers per blood volume (supplemental Figure 2A-B). Surprisingly, there was a marked increase of myeloid cells in the bone marrow of Btk-deficient mice (Figure 1C-F). This increase was mainly due to increased numbers of granulocytes (Figure 1D,F). Also, in the bone marrow of Xid-mice, a significant increase in the population of neutrophils was found (supplemental Figure 2C-F). In addition, we found elevated numbers of erythrocytes per femur in Btk-deficient and Xid-mice (Figure 1E and supplemental Figure 2E). The enlarged granulocyte and erythrocyte populations caused an increase in absolute bone marrow cell numbers calculated per femur of analyzed mice (Figure 1E-F and supplemental Figure 2E-F).
Frequencies of myeloid and erythroid cell populations in blood and bone marrow of Btk-deficient mice. Blood (A-B) from wild-type (wt) and Btk-deficient mice (Btk-ko) were analyzed using an Animal Blood Counter for differential white blood cell (WBC) counts. Frequencies (A) and cell numbers per volume (B) of lymphocytes, neutrophilic granulocytes, and monocytes are shown. (C) Whole bone marrow (WBM) cells of femurs of wild-type and Btk-ko mice were stained for the expression analysis of surface markers CD11b (myeloid cells), Ter119 (erythrocytes), and B220 (lymphocytes). (D) The myeloid compartment was further analyzed by the expression of CD11b+Gr-1+ (granulocytes) and CD11b+Gr-1− (monocytes). The frequency of cell populations was recalculated for defined cell numbers per femur (E-F). Data presented are the mean values (± SD). *P ≤ 0.05; ***P ≤ 0.0005. n represents the number of biological replicates.
Frequencies of myeloid and erythroid cell populations in blood and bone marrow of Btk-deficient mice. Blood (A-B) from wild-type (wt) and Btk-deficient mice (Btk-ko) were analyzed using an Animal Blood Counter for differential white blood cell (WBC) counts. Frequencies (A) and cell numbers per volume (B) of lymphocytes, neutrophilic granulocytes, and monocytes are shown. (C) Whole bone marrow (WBM) cells of femurs of wild-type and Btk-ko mice were stained for the expression analysis of surface markers CD11b (myeloid cells), Ter119 (erythrocytes), and B220 (lymphocytes). (D) The myeloid compartment was further analyzed by the expression of CD11b+Gr-1+ (granulocytes) and CD11b+Gr-1− (monocytes). The frequency of cell populations was recalculated for defined cell numbers per femur (E-F). Data presented are the mean values (± SD). *P ≤ 0.05; ***P ≤ 0.0005. n represents the number of biological replicates.
Analyses of hematopoietic precursor populations in the bone marrow of Btk-deficient mice
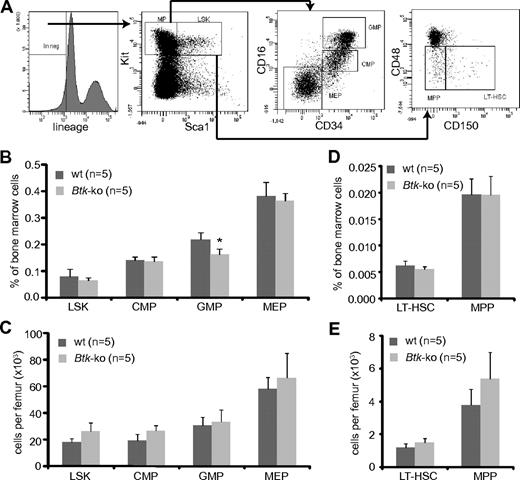
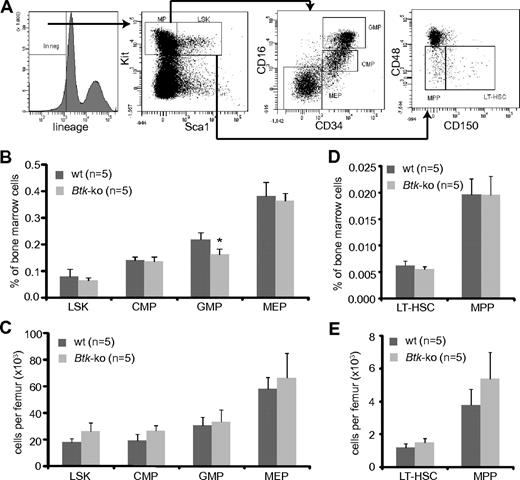
Myeloid progenitors originate from hematopoietic stem cells and reside in the Lin−Sca1−Kit+ population. CMP, MEP, and GMP could be distinguished according to the FcγRII/III and CD34 expression levels20 (Figure 2A). Analyses of myeloid progenitor subpopulations isolated from the bone marrow of Btk-deficient animals revealed a slight but significant reduction of GMP within the bone marrow. Populations of CMP and MEP were similar to those detected in wild-type mice (Figure 2B). However, the absolute numbers of myeloid precursors were comparable with wild-type mice (Figure 2C). Staining of bone marrow cells with antibodies against CD150 and CD4836 (Figure 2A) revealed no significant differences in frequencies, neither in the long-term hematopoietic stem cell compartment (LT-HSC) nor in the compartment of multipotent progenitors (MPP) in Btk-deficient mice (Figure 2D). The absolute numbers of MPP were slightly increased under Btk-deficient conditions (Figure 2E).
Numbers of GMP are reduced in Btk-deficient mice. (A) Bone marrow cells obtained from femurs and tibias were stained for lineage markers, Kit, Sca1, CD34, and FcRγII/III. The Lin−Sca1−Kit+ (MP = myeloid progenitor) population in the bone marrow was further analyzed for myeloid progenitor subpopulations CMP, GMP, and MEP by the expression of CD34 and FcRγII/III, and the LSK population for the expression of CD48 and CD150. Cells from the bone marrow of wild-type (wt) and Btk-ko mice were stained and analyzed by flow cytometry. Hematopoietic precursor subpopulations are shown as percentage of whole bone marrow cells (B,D) or as absolute numbers per femur (C,E). Data presented are mean values (± SD). *P ≤ 0.05. n represents the number of biological replicates.
Numbers of GMP are reduced in Btk-deficient mice. (A) Bone marrow cells obtained from femurs and tibias were stained for lineage markers, Kit, Sca1, CD34, and FcRγII/III. The Lin−Sca1−Kit+ (MP = myeloid progenitor) population in the bone marrow was further analyzed for myeloid progenitor subpopulations CMP, GMP, and MEP by the expression of CD34 and FcRγII/III, and the LSK population for the expression of CD48 and CD150. Cells from the bone marrow of wild-type (wt) and Btk-ko mice were stained and analyzed by flow cytometry. Hematopoietic precursor subpopulations are shown as percentage of whole bone marrow cells (B,D) or as absolute numbers per femur (C,E). Data presented are mean values (± SD). *P ≤ 0.05. n represents the number of biological replicates.
Btk-deficiency impairs myeloid cell development
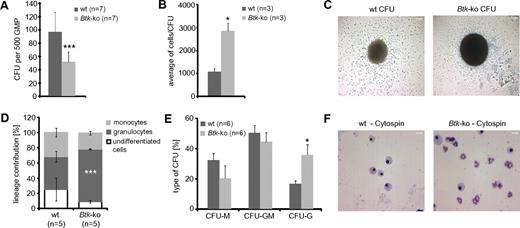
To analyze the basis of the alterations in the myeloid compartment of Btk-deficient and Xid-mice, the developmental capacity of purified Btk-deficient GMP was tested in vitro. Equal numbers of wild-type and Btk-deficient GMP were plated in methylcellulose medium containing SCF, IL-3, and GM-CSF. After 6 days, the frequencies of colony forming units (CFU) were reduced by approximately 50% in Btk-deficient GMP compared with wild-type GMP (Figure 3A). Interestingly, the cell numbers per colony were more than 2-fold higher when Btk was not expressed (Figure 3B-C). Analyses of the phenotype of generated cells originating from Btk-deficient GMP by FACS revealed an increase in granulopoiesis at the expense of monocytes or undifferentiated myeloid cells (Figure 3D). Also, analysis of cell morphology of individual CFU in the absence of Btk revealed an increase of CFU consisting mainly of granulocytes (purity > 90%) at the expense of CFU formed mainly by monocytes/macrophages (Figure 3E-F).
GMP deficient for Btk exhibit a decreased differentiation potential and drive myeloid development toward the granulocytic lineage. A total of 500 GMP sorted out of individual wild-type (wt) and Btk-ko mice were seeded in methylcellulose media supplemented with SCF, IL-3, and GM-CSF. The experiment was performed in quadruplicate. (A) After 6 days in culture, colony-forming units (CFU) were counted. Data presented are the mean values (± SD). (B) The cell number per CFU was calculated. (C) One CFU with a representative size generated from wild-type (wt) or Btk-deficient GMP was photographed and is presented. Scale bars represent 200 μm. (D) Differentiated cells were analyzed by flow cytometry at day 8 of culture. Data presented are the mean (± SD) of cell numbers positive either for the surface marker CD11b+/Gr-1−/F4/80− (undifferentiated cells), for CD11b+/Gr-1+ (granulocytes), or for CD11b+/F4/80+ (monocytes). (E) Twenty to thirty individual CFU per biological replicate were processed for cytospins and Pappenheim staining and analyzed for the cell content by morphology. CFU-M corresponds to more than 90% of the cells were macrophages, CFU-G corresponds to more than 90% of cells were neutrophils, CFU-GM corresponds to the content of macrophages and granulocytes was in between 20 to 80%. (F) A representative cytospin per analyzed genotype is presented. Scale bar represents 50 μm. In panel E the data are presented as mean values (± SEM). Data were analyzed with Student t test. *P ≤ 0.05, ***P ≤ 0.0005. n represents the number of biological replicates.
GMP deficient for Btk exhibit a decreased differentiation potential and drive myeloid development toward the granulocytic lineage. A total of 500 GMP sorted out of individual wild-type (wt) and Btk-ko mice were seeded in methylcellulose media supplemented with SCF, IL-3, and GM-CSF. The experiment was performed in quadruplicate. (A) After 6 days in culture, colony-forming units (CFU) were counted. Data presented are the mean values (± SD). (B) The cell number per CFU was calculated. (C) One CFU with a representative size generated from wild-type (wt) or Btk-deficient GMP was photographed and is presented. Scale bars represent 200 μm. (D) Differentiated cells were analyzed by flow cytometry at day 8 of culture. Data presented are the mean (± SD) of cell numbers positive either for the surface marker CD11b+/Gr-1−/F4/80− (undifferentiated cells), for CD11b+/Gr-1+ (granulocytes), or for CD11b+/F4/80+ (monocytes). (E) Twenty to thirty individual CFU per biological replicate were processed for cytospins and Pappenheim staining and analyzed for the cell content by morphology. CFU-M corresponds to more than 90% of the cells were macrophages, CFU-G corresponds to more than 90% of cells were neutrophils, CFU-GM corresponds to the content of macrophages and granulocytes was in between 20 to 80%. (F) A representative cytospin per analyzed genotype is presented. Scale bar represents 50 μm. In panel E the data are presented as mean values (± SEM). Data were analyzed with Student t test. *P ≤ 0.05, ***P ≤ 0.0005. n represents the number of biological replicates.
In addition to signaling via the GM-CSF receptor, myelopoiesis is driven by signals via TLR.37 This prompted us to investigate the developmental capacity of Btk-deficient GMP in response to the LPS and Pam3CSK4 ligands for TLR4 and TLR2/6, respectively (supplemental Figure 3). Again, lower numbers of colonies but increased numbers of cells per colony were observed when Btk-deficient GMP were seeded (supplemental Figure 3A-B,E-G). Phenotypic analysis of the resulting colonies again revealed a dramatic shift toward granulocytes and away from monocytes and undifferentiated myeloid cells in the absence of Btk (supplemental Figure 3C-D,H).
Impaired maturation of Btk-deficient bone marrow neutrophils
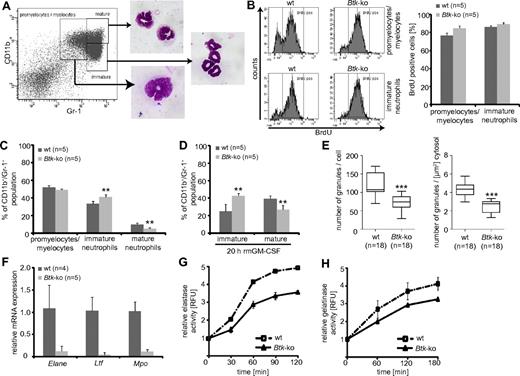
The enhanced granulopoiesis in the bone marrow of Btk-deficient animals, as well as in differentiating Btk-deficient GMP in vitro, could be caused by a higher proliferation rate of either myeloid precursors or developing neutrophils in the bone marrow. In vivo BrdU incorporation assays revealed a slightly enhanced (by approximately 8%) BrdU-incorporation in Btk-deficient promyelocytes/myelocytes that could be distinguished according their expression of CD11b and Gr-138 (Figure 4A-B). In addition, a moderately (5%) higher proliferation rate could be observed within the LSK fraction of Btk-deficient mice, possibly due to higher proliferation of MPP (data not shown; Figure 2E). In addition, we observed severe defects in the basal and GM-CSF–induced maturation of bone marrow neutrophils in the absence of Btk (Figure 4C-D). Maturation of neutrophils is associated with the expression of granule proteins and the appearance of granules. Transmission electron microscopy of Btk-deficient neutrophils revealed a significant decrease in the numbers of granules per cell or cytoplasm (Figure 4E and supplemental Figure 4) which are at the same time of lower electron density. This is a further indication of less granule protein content and immaturity. In line with these observations, we found a clearly reduced expression of elastase, lactoferrin, and myeloperoxidase at the mRNA level in Btk-deficient neutrophils (Figure 4F), which led to an impaired release of elastase and also gelatinase upon immune complex (IC)-induced degranulation (Figure 4G-H).
Impaired maturation of Btk-deficient bone marrow derived neutrophils. (A) The maturation status of granulocytes in the bone marrow can be distinguished by the expression of CD11b and Gr-1. To prove the settings, gated cells were sorted and Pappenheim-staining on cytospins were performed. (B) Mice were treated with BrdU for 4 days and the Gr-1+ subpopulations, according to panel A, were analyzed for BrdU incorporation. (C) The granulocyte maturation phenotype of wild-type (wt) or Btk-deficient (Btk-ko) mice was analyzed on freshly isolated and erythrocytes-depleted bone marrow cells or (D) on for 20 hours in the presence of GM-CSF-matured bone marrow-derived neutrophils. The degree of maturation was defined as depicted in panel A. The percentage of immature and mature neutrophils relative to the overall CD11b+/Gr-1+ population is shown. (E) Wild-type and Btk-deficient bone marrow-derived granulocytes were analyzed by electron microscopy. Granules were counted per cell and per μm2 cytosol using ImageJ software. (F) The expression of granule proteins was analyzed by quantitative PCR (Elane = neutrophils elastase, Ltf = lactotransferrin precursor, Mpo = myeloperoxidase) relative to the expression of β-actin (Actb). The release of elastase (G) or gelatinase (H) upon IC-induced degranulation of wild-type or Btk-deficient granulocytes is presented. Data presented are the mean values (± SD). **P ≤ 0.005; ***P ≤ 0.0005. n represents the number of biological replicates.
Impaired maturation of Btk-deficient bone marrow derived neutrophils. (A) The maturation status of granulocytes in the bone marrow can be distinguished by the expression of CD11b and Gr-1. To prove the settings, gated cells were sorted and Pappenheim-staining on cytospins were performed. (B) Mice were treated with BrdU for 4 days and the Gr-1+ subpopulations, according to panel A, were analyzed for BrdU incorporation. (C) The granulocyte maturation phenotype of wild-type (wt) or Btk-deficient (Btk-ko) mice was analyzed on freshly isolated and erythrocytes-depleted bone marrow cells or (D) on for 20 hours in the presence of GM-CSF-matured bone marrow-derived neutrophils. The degree of maturation was defined as depicted in panel A. The percentage of immature and mature neutrophils relative to the overall CD11b+/Gr-1+ population is shown. (E) Wild-type and Btk-deficient bone marrow-derived granulocytes were analyzed by electron microscopy. Granules were counted per cell and per μm2 cytosol using ImageJ software. (F) The expression of granule proteins was analyzed by quantitative PCR (Elane = neutrophils elastase, Ltf = lactotransferrin precursor, Mpo = myeloperoxidase) relative to the expression of β-actin (Actb). The release of elastase (G) or gelatinase (H) upon IC-induced degranulation of wild-type or Btk-deficient granulocytes is presented. Data presented are the mean values (± SD). **P ≤ 0.005; ***P ≤ 0.0005. n represents the number of biological replicates.
Defective neutrophil function of Btk-deficient mice in the acute inflammatory response
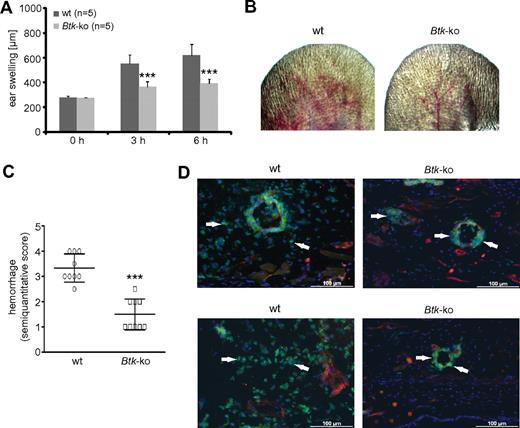
Because we observed maturation defects of Btk-deficient granulocytes, we wondered whether they are functionally relevant. To test this issue in an in vivo approach, we performed the reverse-passive Arthus reaction in the ear (Figure 5). Neutrophils have been shown to be critical for vascular destruction in this IC-mediated vasculitis model.27 Upon initiation of the Arthus reaction, wild-type mice showed a normal response, with edema formation measured as swelling of the treated ear (Figure 5A), petechiae, and hemorrhagic infiltration (Figure 5B-C) after 3 hours. The signs of the inflammatory response, particularly edema and hemorrhage, increased over time in wild-type mice. In contrast, Btk-deficient mice showed significantly fewer signs of IC-mediated vascular destruction (Figure 5A-C). Immunofluorescence analyses revealed that Btk-deficient neutrophils did not migrate into the inflamed tissue in comparison to wild-type neutrophils, although they were attracted and adhered to the luminal site of the blood vessel endothelium, and some also transmigrated through the endothelium (Figure 5D arrowheads).
In Btk-deficient mice, the deposition of IC does not lead to tissue infiltration by neutrophils or vascular destruction. The Arthus reaction was elicited in the left ear of wild-type (wt) or Btk-deficient (Btk-ko) mice. (A) By measuring ear thickness, ear swelling was assessed as a parameter of edema and infiltration. Data presented are mean values of ear thickness (± SD). (B) After 8 hours, pictures of the treated ears of wild-type (wt) or Btk-deficient (Btk-ko) mice were taken for (C) the semiquantitative scoring of petechiae and hemorrhage as a marker of progressive, extensive vascular damage. (D) Eight hours after the Arthus reaction was elicited in wild-type (wt) and Btk-deficient (Btk-ko) mice, ears were harvested, embedded, and processed for immunofluorescence analyses. Slides were stained with anti–Gr-1-FITC, anti-CD31+anti-PE, and DAPI. Arrowheads indicate the position of neutrophils.
In Btk-deficient mice, the deposition of IC does not lead to tissue infiltration by neutrophils or vascular destruction. The Arthus reaction was elicited in the left ear of wild-type (wt) or Btk-deficient (Btk-ko) mice. (A) By measuring ear thickness, ear swelling was assessed as a parameter of edema and infiltration. Data presented are mean values of ear thickness (± SD). (B) After 8 hours, pictures of the treated ears of wild-type (wt) or Btk-deficient (Btk-ko) mice were taken for (C) the semiquantitative scoring of petechiae and hemorrhage as a marker of progressive, extensive vascular damage. (D) Eight hours after the Arthus reaction was elicited in wild-type (wt) and Btk-deficient (Btk-ko) mice, ears were harvested, embedded, and processed for immunofluorescence analyses. Slides were stained with anti–Gr-1-FITC, anti-CD31+anti-PE, and DAPI. Arrowheads indicate the position of neutrophils.
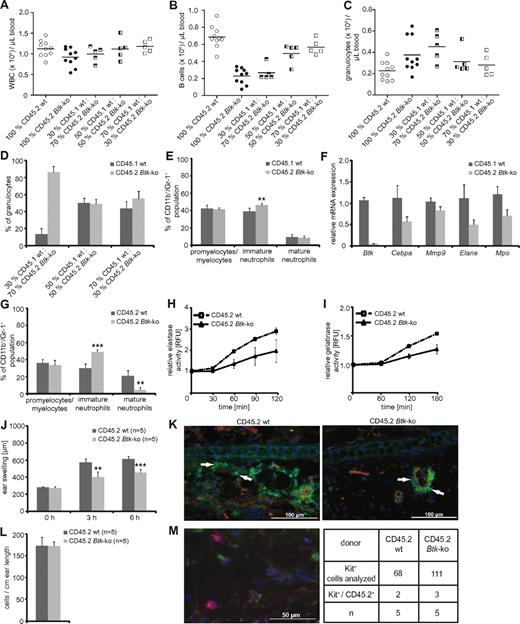
To investigate whether Btk plays a cell-intrinsic role in neutrophil development and function, syngeneic transplantation experiments were performed using CD45.2+ bone marrow obtained from wild-type or Btk-deficient mice, which was transplanted into CD45.1+ lethally irradiated mice with or without syngeneic bone marrow competitor cells in different ratios. Blood analyses were performed 4 weeks after transplantation. Mice that had received Btk-deficient bone marrow showed reduced numbers of white blood counts per μL blood due to lower numbers of B cells, similar to Btk-deficient mice; the B-cell reduction could be corrected by increasing the amount of wild-type bone marrow cells transplanted. In contrast to Btknull mice, we found a marked increase in the number of granulocytes in the blood when Btk-deficient bone marrow was transplanted. Increasing amounts of wild-type bone marrow rescued this phenotype (Figure 6A-C). Next, we examined the bone marrow of recipient mice 6 weeks after transplantation. Transplantation of wild-type and Btk-deficient bone marrow at different ratios again revealed that the absence of Btk favored granulopoiesis (Figure 6D). However, Btk-deficient granulocytes exhibited an immature phenotype, determined by the expression levels of CD11b/Gr-1 (Figure 6E,G), as well as the expression of the lineage-determining transcription factor C/EBPα, the presence of granule proteins gelatinase, elastase, and myeloperoxidase (Figure 6F), or by the release of elastase and gelatinase in an IC-induced degranulation assay (Figure 6H-I).
The maturation and functional defects of Btk-deficient neutrophils are neutrophil-intrinsic. Chimeric mice were generated by transplanting either CD45.2+ wild-type or Btk-deficient bone marrow into CD45.1+ lethally irradiated mice. Alternatively, CD45.1+ lethally irradiated mice received a mixture of CD45.1+ wild-type and CD45.2+ Btk-deficient bone marrow in different ratios. (A-C) Blood analyses were performed 4 weeks after transplantation. The bone marrow of CD45.1/CD45.2 chimeras was analyzed 6 weeks after transplantation for (D) the reconstitution of the neutrophil compartment and (E) the maturation status of the CD11b/Gr-1 population. CD45.1+ wild-type or CD45.2+ Btk-deficient granulocytes were sorted out of the bone marrow of chimeric mice and analyzed for the expression of Btk as a control and for C/EBPα (Cebpa), gelatinase (Mmp9), elastase (Elane), and myeloperoxidase (Mpo) by quantitative PCR (F). Granulocytes obtained from CD45.1+ lethally irradiated mice that received bone marrow from CD45.2+ wild-type (wt) or Btk-deficient (Btk-ko) mice were analyzed for (G) the maturation status of the CD11b/Gr-1 population, as well as the release of elastase (H) or gelatinase (I) in an immune-complex/Fc-mediated degranulation assay. CD45.1+ lethally irradiated mice that received bone marrow from CD45.2+ wild-type (wt) or Btk-deficient mice (Btk-ko) were investigated in the IC-mediated Arthus reaction (J) for ear swelling as a sign of tissue damage and edema formation. Treated ears were harvested 8 hours after eliciting the immune response and analyzed by immunofluorescence (K); red = CD31; green = Gr-1, arrowheads; blue = DAPI. (L) In parallel, one-half of the treated ears were analyzed by toluidin-staining for the presence of mast cells. (M) To determine the origin of mast cells, ear slides were analyzed by anti-CD45.2 (green; corresponds to donor cells), anti-Kit (red), and DAPI (blue). A representative image taken from an ear of a lethally irradiated CD45.1+ mouse that received CD45.2+ Btk-deficient bone marrow cells is presented. n represents the number of biological replicates.
The maturation and functional defects of Btk-deficient neutrophils are neutrophil-intrinsic. Chimeric mice were generated by transplanting either CD45.2+ wild-type or Btk-deficient bone marrow into CD45.1+ lethally irradiated mice. Alternatively, CD45.1+ lethally irradiated mice received a mixture of CD45.1+ wild-type and CD45.2+ Btk-deficient bone marrow in different ratios. (A-C) Blood analyses were performed 4 weeks after transplantation. The bone marrow of CD45.1/CD45.2 chimeras was analyzed 6 weeks after transplantation for (D) the reconstitution of the neutrophil compartment and (E) the maturation status of the CD11b/Gr-1 population. CD45.1+ wild-type or CD45.2+ Btk-deficient granulocytes were sorted out of the bone marrow of chimeric mice and analyzed for the expression of Btk as a control and for C/EBPα (Cebpa), gelatinase (Mmp9), elastase (Elane), and myeloperoxidase (Mpo) by quantitative PCR (F). Granulocytes obtained from CD45.1+ lethally irradiated mice that received bone marrow from CD45.2+ wild-type (wt) or Btk-deficient (Btk-ko) mice were analyzed for (G) the maturation status of the CD11b/Gr-1 population, as well as the release of elastase (H) or gelatinase (I) in an immune-complex/Fc-mediated degranulation assay. CD45.1+ lethally irradiated mice that received bone marrow from CD45.2+ wild-type (wt) or Btk-deficient mice (Btk-ko) were investigated in the IC-mediated Arthus reaction (J) for ear swelling as a sign of tissue damage and edema formation. Treated ears were harvested 8 hours after eliciting the immune response and analyzed by immunofluorescence (K); red = CD31; green = Gr-1, arrowheads; blue = DAPI. (L) In parallel, one-half of the treated ears were analyzed by toluidin-staining for the presence of mast cells. (M) To determine the origin of mast cells, ear slides were analyzed by anti-CD45.2 (green; corresponds to donor cells), anti-Kit (red), and DAPI (blue). A representative image taken from an ear of a lethally irradiated CD45.1+ mouse that received CD45.2+ Btk-deficient bone marrow cells is presented. n represents the number of biological replicates.
Chimeric mice receiving 100% wild-type or Btk-deficient bone marrow cells were examined in the reverse-passive Arthus reaction. Similar to our previous findings using wild-type and Btk-deficient mice, we observed less edema formation, and no tissue-infiltrating neutrophils when Btk-deficient bone marrow cells were transplanted into wild-type recipients (Figure 6J-K). To exclude any contribution of functionally impaired Btk-deficient mast cells, mast cell numbers and origin were analyzed histologically 8 hours after eliciting the IC-mediated immune response. This analyses revealed equal numbers of residing mast cells in the ear that were 97% of host origin (Figure 6L-M). Thus, skin mast cells had remained Btk wild-type. Together, these experiments demonstrate that the defect observed in Btk-deficient neutrophils is not impacted by extrinsic factors such as the bone marrow microenvironment or by the compromised B cell compartment. Because we observed a severely impaired Arthus reaction in the presence of Btk-deficient neutrophils and Btk-proficient mast cells, we conclude that Btk-deficient neutrophils are not able to mount an efficient IC-induced immune response in vivo and that this defect is intrinsic to neutrophils.
Molecular mechanisms leading to the impaired development and function of Btk-deficient granulocytes
Given the defects in GM-CSF-induced differentiation in the absence of Btk, we wondered whether Btk is involved in GM-CSF signaling. Indeed, we found that Btk is phosphorylated in wild-type GM-CSF-treated neutrophils (Figure 7A).
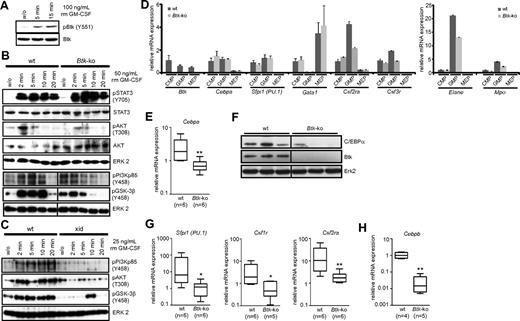
Btk is involved in GM-CSF–mediated signaling and is necessary for the sufficient expression of transcription factors important for efficient granulopoiesis. (A) MACS-sorted CD11b+ cells from the bone marrow of wild-type (wt) mice were treated for the indicated time with GM-CSF. Cells were lysed and analyzed by immunoblotting for the phosphorylation of Btk at Y551 (Btk pY551). The membrane was then stripped and analyzed for the amount of total Btk protein loaded onto the gel (Btk). (B-C) Erythrocytes-depleted bone marrow cells of wild-type (wt) or Btk-deficient (Btk-ko) mice or Xid mice (xid) were treated for the indicated time with GM-CSF. Cells were lysed and analyzed by immunoblotting for the phosphorylation of Stat3 (pStat3), Akt (pAkt), PI3K (pPI3Kp85), and GSK-3β (pGSK-3β), as well as for the total amounts of Stat3, Akt, and Erk2 proteins used for analyses. (D) CMP, GMP, and MEP were isolated from the bone marrow of wild-type (wt) and Btk-deficient (Btk-ko) mice by FACS. RNA was prepared and analyzed for the mRNA expression of Btk (Btk), C/EBPα (Cebpa), PU.1 (Sfpi1), GATA1 (Gata1), and target genes like GM-CSF-Rα (Csf2ra), G-CSF-R (Csf3r), elastase (Elane), and myeloperoxidase (Mpo) by real-time PCR relative to the expression of Hprt. (E) Total bone marrow cells were isolated from wild-type (wt) and Btk-deficient (Btk-ko) mice. RNA was prepared and analyzed for the mRNA expression of C/EBPα (Cebpa) relative to the expression of Gapdh. (F) Bone marrow cells of wild-type and Btk-deficient mice were lysed and analyzed for the expression of C/EBPα. As a control for the genotype of mice used, a Western blot was performed using an anti-Btk antibody. Probing of the membrane with an anti Erk2-antibody served as loading control. (G) Total bone marrow cells were isolated from wild-type (wt) and Btk-deficient (Btk-ko) mice. RNA was prepared and analyzed for the mRNA expression of PU.1 (Sfpi1), M-CSF-R (Csf1r), and GM-CSF-Rα (Csf2ra) relative to the expression of Gapdh. (H) Bone marrow-derived neutrophils were isolated from wild-type (wt) and Btk-deficient (Btk-ko) mice and analyzed for the expression of C/EBPβ (Cebpb) relative to the expression of β-actin (Actb). Data presented are mean values (± SD). *P ≤ 0.05, **P ≤ 0.005. n represents the number of biological replicates. (B,F) Vertical lines have been inserted to indicate a repositioned gel lane.
Btk is involved in GM-CSF–mediated signaling and is necessary for the sufficient expression of transcription factors important for efficient granulopoiesis. (A) MACS-sorted CD11b+ cells from the bone marrow of wild-type (wt) mice were treated for the indicated time with GM-CSF. Cells were lysed and analyzed by immunoblotting for the phosphorylation of Btk at Y551 (Btk pY551). The membrane was then stripped and analyzed for the amount of total Btk protein loaded onto the gel (Btk). (B-C) Erythrocytes-depleted bone marrow cells of wild-type (wt) or Btk-deficient (Btk-ko) mice or Xid mice (xid) were treated for the indicated time with GM-CSF. Cells were lysed and analyzed by immunoblotting for the phosphorylation of Stat3 (pStat3), Akt (pAkt), PI3K (pPI3Kp85), and GSK-3β (pGSK-3β), as well as for the total amounts of Stat3, Akt, and Erk2 proteins used for analyses. (D) CMP, GMP, and MEP were isolated from the bone marrow of wild-type (wt) and Btk-deficient (Btk-ko) mice by FACS. RNA was prepared and analyzed for the mRNA expression of Btk (Btk), C/EBPα (Cebpa), PU.1 (Sfpi1), GATA1 (Gata1), and target genes like GM-CSF-Rα (Csf2ra), G-CSF-R (Csf3r), elastase (Elane), and myeloperoxidase (Mpo) by real-time PCR relative to the expression of Hprt. (E) Total bone marrow cells were isolated from wild-type (wt) and Btk-deficient (Btk-ko) mice. RNA was prepared and analyzed for the mRNA expression of C/EBPα (Cebpa) relative to the expression of Gapdh. (F) Bone marrow cells of wild-type and Btk-deficient mice were lysed and analyzed for the expression of C/EBPα. As a control for the genotype of mice used, a Western blot was performed using an anti-Btk antibody. Probing of the membrane with an anti Erk2-antibody served as loading control. (G) Total bone marrow cells were isolated from wild-type (wt) and Btk-deficient (Btk-ko) mice. RNA was prepared and analyzed for the mRNA expression of PU.1 (Sfpi1), M-CSF-R (Csf1r), and GM-CSF-Rα (Csf2ra) relative to the expression of Gapdh. (H) Bone marrow-derived neutrophils were isolated from wild-type (wt) and Btk-deficient (Btk-ko) mice and analyzed for the expression of C/EBPβ (Cebpb) relative to the expression of β-actin (Actb). Data presented are mean values (± SD). *P ≤ 0.05, **P ≤ 0.005. n represents the number of biological replicates. (B,F) Vertical lines have been inserted to indicate a repositioned gel lane.
One of the early events upon GM-CSF receptor ligation is the phosphorylation and activation of Stat3. Whereas Stat3 phosphorylation was unchanged in Btk-deficient neutrophils, the phosphorylation of PI3-kinase, Akt, and GSK-3β, which is important for the regulation of C/EBPα activity, clearly depended on the presence of active Btk. These data were confirmed using lysates prepared from granulocytes obtained from Xid-mice (Figure 7B-C) and strongly suggest the importance of Btk in GM-CSF–mediated signaling in neutrophils.
To gain more insight into the molecular network that guides myelopoiesis, and particularly the involvement of Btk, we analyzed the expression of Btk in different myeloid progenitors (Figure 7D). The highest Btk expression was detected in CMP; however, considerable amounts of Btk were also detectable in MEP and GMP. In parallel, we assessed the expression levels of Cebpa, Sfpi1, and Gata1, which are the main transcription factors regulating myeloid cell fate decisions. Interestingly, we were not able to detect gross alterations between wild-type and Btk-deficient CMP or GMP with respect to Cebpa and Sfpi1 expression. However, the RNA expression level of Csf2ra (GM-CSF-Rα)—a known target of Sfpi1—was reduced in Btk-deficient GMP. Also, the expression levels of Csf3r (G-CSF-R), elastase, and myeloperoxidase were clearly reduced in Btk-deficient GMP (Figure 7D). However, when the whole bone marrow of wild-type and Btk-deficient mice was analyzed, a clear reduction in the expression of Cebpa (5-fold) at RNA and protein level could be detected (Figure 7E-F). In addition, a drastic reduction in Sfpi1 expression (25-fold) was observed in the absence of Btk. In line with these findings, the expression of known Sfpi1 target genes, such as Csf1r (6-fold) and Csf2ra (10-fold), was clearly down-regulated when Btk-deficient bone marrow was analyzed (Figure 7G).
In addition, the RNA expression of Cebpb (C/EBPβ), another important transcription factor in neutrophil maturation, particularly granule protein expression,24 is also clearly down-regulated in neutrophils purified from the bone marrow of Btk-deficient mice (Figure 7H).
We wondered how stimuli, like LPS, that mimic a bacterial infection might influence myelopoiesis under Btk-deficient conditions. In vitro, LPS induced an enhanced granulopoiesis when the whole bone marrow (supplemental Figure 5A) or isolated myeloid progenitors (supplemental Figure 3A-D) obtained from Btk-deficient animals are treated. Similar to GM-CSF treatment, LPS did not significantly change the numbers of circulating granulocytes in comparison to untreated control mice (supplemental Figure 5B-C). However, LPS led to a strong increase of Btk-deficient bone marrow neutrophils in comparison to wild-type LPS treated animals, as well as to the untreated controls (supplemental Figure 5D). The maturation status of granulocytes, as well as the percentage of progenitor subpopulations (supplemental Figure 5E-F) in the bone marrow of Btk-deficient LPS-treated mice were generally comparable with those of wild-type animals. However, Cebpa expression, which is drastically induced in the GMP of both wild-type and Btk-deficient animals, was significantly reduced by approximately 30% in the absence of functional Btk (supplemental Figure 5G). The same held true for the expression of gelatinase, which was decreased by 50% in the absence of Btk. The expression of elastase was comparable in wild-type and Btk-deficient GMP (supplemental Figure 5H-I).
Neutropenia in patients suffering from XLA can be rescued by G-CSF treatment. Therefore, we asked whether this G-CSF–induced granulopoiesis is efficient in Btk-deficient mice. Similar to humans affected by XLA, in vivo administration of G-CSF led to an increase of circulating granulocytes in Btk-deficient mice to levels similar to those observed in wild-type mice (supplemental Figure 5B-C). In contrast to peripheral blood, G-CSF administration strongly induced an increase in the number of bone marrow neutrophils in wild-type but not in Btk-deficient mice. In addition, the Btk-deficient granulocytes exhibited a more immature phenotype in comparison to wild-type mice (supplemental Figure 5D-E).
After G-CSF treatment, the numbers of GMP in Btk-deficient mice were considerably reduced in comparison to wild-type animals (supplemental Figure 5F). In addition, G-CSF did not rescue the reduced expression of Cebpa in Btk-deficient GMP or its targets, elastase and gelatinase (supplemental Figure 5G-I). These results suggest that G-CSF, as well as LPS, induces the development of functionally impaired granulocytes in Btk-deficient mice.
Discussion
The most striking defect with regard to myeloid development in Btk-deficient mice was the increase of granulopoiesis in vitro and in vivo. However, Btk-deficient neutrophils showed defects in GM-CSF-signaling and GM-CSF–induced maturation accompanied with impaired function during an acute inflammatory response. Generation of bone marrow chimeras revealed a cell-intrinsic role of Btk in neutrophil development and function.
Here we show that GMP isolated from the bone marrow of Btk-deficient mice preferentially developed into granulocytes at the expense of monocytes or undifferentiated cells when cultivated in the presence of GM-CSF, SCF, and IL-3 or TLR ligands. In addition, we found enhanced erythropoiesis in the bone marrow of Btk-deficient and Xid-mice. The function of Btk in erythroid progenitors has been analyzed previously.39 The expanded populations of both granulocytes and erythrocytes caused an increase of the total cell number in the bone marrow of Btk-mutant mice. In Btk-deficient and Xid-mice, despite hypercellularity and enhanced granulo- and erythropoiesis in the bone marrow, the numbers of granulocytes and erythrocytes in the blood were not significantly altered in comparison to wild-type littermates. Our data are at odds with results published previously.6 When analyzing Xid-mice, these authors found reduced numbers of neutrophils in the bone marrow as well as in the peripheral blood. However, in our analyses, the numbers of neutrophils and macrophages were significantly reduced in spleens of Btknull mice.
Although we found an enhanced granulopoiesis under Btk-deficient conditions, the generated granulocytes were less mature. This immature state, defined by the expression levels of CD11b and Gr-1, was associated with an inefficient development of granules as well as the expression of granule proteins, like elastase, myeloperoxidase, lactoferrin, or gelatinase, which ultimately impaired the function of neutrophilic granulocytes.
Cell fate decision is a regulated process and depends on the expression level of certain transcription factors that in turn are responsible for cell type-specific gene expression. Two transcription factors are primarily involved in myeloid lineage specification: C/EBPα and PU.1.22,23 Although the expression of C/EBPα was comparable between wild-type and Btk-deficient CMP and GMP, the C/EBPα expression level was clearly reduced when the whole bone marrow of Btk-deficient mice was analyzed. Btk does not only influence the expression level of C/EBPα. Here, we showed that Btk is involved in GM-CSF–induced signaling events regulating C/EBPα activity. Upon GM-CSF treatment, the phosphorylation of PI3-kinase, as well its target Akt, was severely impaired in Btk-deficient neutrophils. Modulation of Akt activity influences myelopoiesis.40 Akt phosphorylates GSK-3β, which in turn inhibits GSK function. Active GSK-3β phosphorylates C/EBPα on Thr222/226, leading to the modulation of C/EBPα activity.40 Therefore, reduced phosphorylation of Akt could be responsible for an inefficient inactivation of GSK3, also observed in Btk-deficient granulocytes, that leads finally to the alteration of C/EBPα function and consequently to changes in C/EBPα target gene expression. C/EBPα binds and activates, among others, the PU.1 promoter and its distal enhancer.41-43
The Ets-family transcription factor PU.1 has been shown to be critically important for myeloid development. Conditional PU.1 inactivation in GMP led to enhanced granulopoiesis, suggesting specific functions of PU.1 during myeloid development.44 In addition, the level of PU.1 expression is important for the development of granulocytes versus monocytes: low levels of PU.1 induce granulopoiesis, whereas high levels drive the development of monocytes.45,46 Here, we described that PU.1 expression was normal in CMP and GMP. However, the expression of the GM-CSF-Rα mRNA, a known PU.1 target, was reduced at the level of GMP. This might indicate that the PU.1 function is already altered at that level of GMP. However, the mRNA expression of PU.1, as well as its targets GM-CSF-Rα and M-CSF-R, were found to be expressed at significantly lower levels in total bone marrow cells of Btk-mutant mice. Therefore, reduced levels of PU.1 and, consequently, its targets like M-CSFR and GM-CSFR in the bone marrow of Btk-deficient mice could contribute to the enhanced granulopoiesis observed in Btk-deficient mice as well as the GM-CSF–induced development of Btknull GMP.
Interestingly, TLR-induced myeloid differentiation of Btk-deficient GMP led to enhanced granulopoiesis at the expense of monocytes/macrophages. As TLR-induced myeloid differentiation of precursor cells obviates the need for growth and differentiation factors like M-CSF and GM-CSF,37 it is clear that developmental machinery is induced similar to the one activated upon GM-CSF treatment.
In addition, our study reveals severe functional defects of Btk-deficient neutrophils. Using the reverse-passive Arthus reaction as a model of an acute IC-mediated inflammatory response, we observed less vascular destruction as evidenced by less edema formation, and reduced amounts of petechiae and hemorrhage in Btk-deficient mice. Here we show that IC-induced Btk-deficient neutrophils release significantly reduced levels of elastase and gelatinase, enzymes implicated in endothelial damage. Analyses of mRNA expression levels of elastase and other primary, secondary, and tertiary granule enzymes revealed that Btk is necessary for the sufficient expression not only of elastase, but also of other enzymatic active proteins, like myeloperoxidase, lactotransferrin precursor, gelatinase, and neutrophilic granule protein. Therefore, the reduced expression of granule proteins could lead to the reduced tissue damage observed during the Arthus reaction in Btk-deficient animals.
Certainly, other factors could also contribute to the observed inefficient immune response, such as impaired recruitment to the side of inflammation, as previously shown in Xid-mice treated with oyster glycogen or thioglycollate,6 or the impaired E-selectin–mediated slow rolling of Btk-deficient neutrophils.47 However, we could detect Btk-deficient neutrophils attached to the blood vessel endothelium as well as neutrophils that were able to transmigrate into the perivascular space. Hence, additional factors such as reduced enzyme expression and consequent degranulation defects likely contribute to the observed phenotype of Btk-deficient mice in the Arthus reaction.
Most likely, the impaired expression or function/modification of the transcription factors C/EBPα and C/EBPβ in the bone marrow, which are mainly necessary for granule protein expression,23 caused defective expression of granule content in Btk-deficient PMN. Because granule enzymes are necessary for bacterial and fungal killing, these data suggest that Btk-deficient neutrophils are severely compromised in the efficient elimination of invading pathogens.
Moreover, whereas C/EBPα is the master regulator for the basal granulopoiesis,22,23 C/EBPβ is necessary for cytokine or infection-induced granulopoiesis in vitro as well in vivo.25 Cytokines like GM-CSF, G-CSF, and IL-3 are responsible for the up-regulation of C/EBPβ in neutrophils, and C/EBPβ-deficient progenitors failed to generate efficient granulopoiesis in response to signals that mimic an acute infection. Here, we have shown that Btk is involved in GM-CSF–mediated signaling of neutrophils and that C/EBPα and C/EBPβ expression levels are significantly reduced in Btk-deficient bone marrow cells. These data indicate that Btk plays an important role for the development, maturation, and function of neutrophilic granulocytes. Thus, neutropenia that has been reported in XLA patients3,19 might be associated with cell-intrinsic neutrophil dysfunction that in turn could contribute to the severity of bacterial infections reported in these cases. Because neutropenia in XLA patients has been associated with mutations within the BTK gene that cause the loss of Btk expression or function,19 it is likely that the type of BTK gene mutation may be associated with the degree of impaired granulopoiesis.
In addition, our data indicate that Btk-deficient granulocytes are severely impaired functionally during an acute inflammatory response induced by either IC or LPS. Therefore, the function of neutrophils of XLA patients should be reassessed under conditions mimicking an acute infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are very grateful to T. Wirth (Institute of Physiological Chemistry, Ulm University) for intensive discussions and scientific support, P. Walther and E. Schmid (Central Electron Microscopy Unit, Ulm University) for their great support in TEM, W. Ellmeier and U. Schmidt (Institute of Immunology, University Vienna) for supporting mouse analyses, P. Weihrich and M. Gerstenlauer for excellent technical assistance, and B. Kraut for help in immunofluorescence analyses. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG, SFB 497) to A.S. (TP C7), H.R.R. (TP B5), and C.B. (TP C9 and BR-2891/2).
Authorship
Contribution: K.F., A.S., G.T., E.K., T.B.F., and L.B. performed experiments; K.F. analyzed results and made the figures; H.R.R. designed the research; and C.B. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cornelia Brunner, Institute of Physiological Chemistry, Ulm University, Albert-Einstein-Allee 11, D-89081 Ulm, Germany; e-mail: cornelia.brunner@uni-ulm.de.