Abstract
Information on natural killer (NK)–cell receptor-ligand interactions involved in the response to human cytomegalovirus (HCMV) is limited and essentially based on the study of infected fibroblasts. Experimental conditions were set up to characterize the NK response to HCMV-infected myeloid dendritic cells (DCs). Monocyte-derived DCs (moDCs) infected by the TB40/E HCMV strain down-regulated the expression of human leukocyte antigen class I molecules and specifically activated autologous NK-cell populations. NKG2D ligands appeared virtually undetectable in infected moDCs, reflecting the efficiency of immune evasion mechanisms, and explained the lack of antagonistic effects of NKG2D-specific monoclonal antibody. By contrast, DNAM-1 and DNAM-1 ligands (DNAM-1L)–specific monoclonal antibodies inhibited the NK response at 48 hours after infection, although the impact of HCMV-dependent down-regulation of DNAM-1L in infected moDCs was perceived at later stages. moDCs constitutively expressed ligands for NKp46 and NKp30 natural cytotoxicity receptors, which were partially reduced on HCMV infection; yet, only NKp46 appeared involved in the NK response. In contrast to previous reports in fibroblasts, human leukocyte antigen-E expression was not preserved in HCMV-infected moDCs, which triggered CD94/NKG2A+ NK-cell activation. The results provide an insight on key receptor-ligand interactions involved in the NK-cell response against HCMV-infected moDCs, stressing the importance of the dynamics of viral immune evasion mechanisms.
Introduction
Human cytomegalovirus (HCMV) infection is highly prevalent in healthy persons and the virus remains in a lifelong latent state, undergoing occasional reactivation and causing an important morbidity in immunocompromised patients.1 An effective defense against CMV infection requires the participation of both T and NK cells.2 To escape T cell-mediated recognition, CMV interferes with the expression of major histocompatibility complex molecules and antigen presentation.3 The loss of human leukocyte antigen (HLA) class I molecules in infected cells impairs the engagement of inhibitory receptors, thus promoting the activation of NK-cell effector functions. Reciprocally, the virus has developed different strategies to escape NK-cell surveillance, preventing the expression of ligands for some activating receptors (ie, NKG2D, DNAM-1)4-9 or selectively maintaining inhibitory receptors for HLA class I molecules engaged. The UL18 glycoprotein binds with high affinity to the LILRB1 inhibitory receptor expressed in different leukocytes10 ; yet, formal experimental evidence for its function in immune evasion remains elusive.11 In the same line, a leader signal peptide of the UL40 HCMV protein was shown to stabilize the surface expression of HLA-E, thus repressing NK-cell activation by engagement of the inhibitory CD94/NKG2A receptor.12,13 On the other hand, the NK-cell subset bearing the CD94/NKG2C triggering molecule tends to expand in peripheral blood from HCMV+ persons14 and on in vitro coculture of peripheral blood mononuclear cells (PBMCs) with HCMV-infected fibroblasts.15 A CD94/NKG2C+ NK lymphocytosis was detected in a patient with a selective T-cell deficiency, coinciding with an acute HCMV infection and associated with a reduction of viremia.16 Altogether, these results suggest that the CD94/NKG2C+ NK-cell subset may play an active role in the response to HCMV.
Information on the nature of NK-cell receptor (NKR)–ligand interactions involved in the response against HCMV-infected cells is scarce, and most studies have been performed in infected fibroblasts, not fully representative of the different cell types susceptible to in vivo infection. Cells of the myeloid lineage are considered reservoirs for HCMV latency, and differentiation of myeloid progenitors to dendritic cells (DCs) may reactivate the virus.17 Furthermore, monocyte-derived DCs (moDCs) appear susceptible to in vitro HCMV infection by endothelial cell (EC)–adapted viral strains.18 Infection of moDCs has been reported to impair their maturation, inhibiting surface expression of major histocompatibility complex class I and II, costimulatory molecules, and chemokine receptors (ie, CCR1 and CCR5), thus interfering with the development of virus-specific T-cell responses.19-21
Beyond their key role in antigen presentation, DCs may establish a cross-talk with NK cells that reciprocally regulates their functions. Myeloid DCs can induce NK-cell functions, and activated NK cells may alternatively kill immature DCs or promote their maturation. These processes are dependent on activating NKR (ie, NKp30 and DNAM-1) and cytokine secretion.22-25 A subset of NK cells expressing the inhibitory CD94/NKG2A receptor but lacking KIR is mainly responsible for mediating the response to autologous immature moDCs.26 HCMV infection of plasmacytoid DCs has been shown to alter plasmacytoid DC-mediated activation of NK cells, inducing CD69 surface expression and interferon-γ (IFN-γ) secretion but decreasing perforin levels and cytotoxicity against the K562 tumor cell line27 ; yet, lysis/degranulation in response to infected plasmacytoid DCs was not assessed, nor were the NK-cell receptors participating in that response characterized.
In the present study, suitable experimental conditions were set up to characterize the NK-cell response against autologous HCMV-infected moDCs. Our results demonstrate that NK cells efficiently react against HCMV-infected moDCs, overcoming viral immune evasion strategies, and furthermore provide novel insights on the role played by different activating NK-cell receptor-ligand interactions, as well as on the influence of viral immune evasion mechanisms in this complex process.
Methods
Cell isolation and generation of moDCs
PBMCs were obtained from heparinized blood samples by separation on Ficoll-Hypaque gradient (Lymphoprep; Axis-Shield PoC AS). Samples were obtained with the informed consent of the subjects in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional Ethics Committee. Standard clinical diagnostic tests were used to analyze serum samples for circulating IgG antibodies against HCMV (Abbott Laboratories).
moDCs were generated as described previously.28 Briefly, monocytes were obtained by positive selection, using anti-CD14 microbeads (Miltenyi Biotec), and cultured for 6 days in RPMI 1640 medium supplemented with 10% fetal calf serum, interleukin-4 (IL-4; 25 ng/mL, R&D Systems), and granulocyte-macrophage colony-stimulating factor (50 ng/mL, PeproTech). After 6 days, cells were CD14−CD1a+ and CD83−, as assessed by immunofluorescence staining.
NK-cell enrichment was performed by negative selection using EasySep Human NK Cell Enrichment kit (StemCell Technologies) according to the manufacturer's recommendations, obtaining more than 98% CD3− CD56+ populations. In some assays, NK cells were activated by culturing PBMCs either overnight or for 7 days, with 40 U/mL or 10 U/mL recombinant IL-2 (Proleukin; Chiron) added every 2 days, respectively.
Antibodies, immunofluorescence, and flow cytometric analysis
Flow cytometry was performed using monoclonal antibodies specific for the following surface molecules: CD14-phycoerythrin (PE), CD3-PE, CD83-PE, CD1a-PE, and CD56-PE (BD Biosciences PharMingen), CD69-PE, CD25-PE, and HLA-ABC-fluorescein isothiocyanate (FITC) (Immunotools), CD94/NKG2C-PE (R&D Systems), and CD94/NKG2A-PE (Beckman Coulter). HP-1F7 anti-HLA class I was generated in our laboratory,28 and D1.12 anti-HLA class II was kindly provided by Dr Roberto Accolla (Università of Insubria, Varese). The HLA-E-specific 3D12 monoclonal antibody (mAb),29 anti-MICA (clone BAM195, IgG1) mAb,30 as well as L95 (IgG1, anti-PVR) and L14 (IgG2a, anti-Nectin-2) mAbs25 were previously described. MICB (clone 236511, IgG2b), ULBP-1 (clone 170818, IgG2a), ULBP-2 (clone 165903, IgG2a), and ULBP-3 (clone 166510, IgG2a) specific mAbs were purchased from R&D Systems; anti-ULBP-4 mAb (clone M475, IgG1) was kindly provided by Amgen. Control IgG2a-PE was from BD Biosciences; FITC- or PE-conjugated F (ab′)2 rabbit antimouse Ig was from Dako Denmark.
Cells were pretreated with human aggregated IgG (10 μg/mL) to block Fc receptors and subsequently labeled with specific antibodies. For indirect immunostaining, samples were incubated with unlabeled antibodies followed, after washing, by FITC- or PE-conjugated F(ab′)2 polyclonal rabbit antimouse Ig. Flow cytometric analysis was performed as previously described.28 Cell viability was measured using the annexin-V-FLUOS Staining Kit (Roche Diagnostics) according to the manufacturer's instructions.
For blocking experiments, the following mAbs were used at saturating concentrations: KL247 (IgM, anti-NKp46), F252 (IgM, anti-NKp30), F5 (IgM, anti-DNAM-1), L95 (IgG1, anti-PVR), L14 (IgG2a, anti-Nectin-2), BAT221 (IgG1, anti-NKG2D); a neutralizing mAb for human IL-12 (clone 20C2, IgG1) was obtained from ATCC and blocking antibody against IFN receptor chain 2 (IFNAR; clone MMHAR-2, IgG2a) was from Calbiochem. An anti-myc mAb (9E10, IgG1) was used as negative control.
Expression of NCR-Fc chimeric constructs and immunofluorescence
The plasmids for expression of NKp30 and NKp46 as fusion proteins with the Fc portion of human IgG1 have been described31 and were generously provided by Dr Ofer Mandelboim (Hebrew University-Hadassah Medical School, Jerusalem, Israel). Recombinant proteins were expressed by transfection of HEK 293T cells using the calcium phosphate method; 18 hours later, cells were washed twice and cultured on serum-free medium (EX-cell ACF CHO Medium, Sigma-Aldrich). At days 3 and 6, the supernatants were recovered and the soluble molecules purified by affinity chromatography with Protein A-Sepharose CL-4B (GE Healthcare) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. The specificity of NCR-Fc chimeric proteins was checked using a competition assay by staining CD3−CD56+ NK cells with NKp46 (BAB281) or NKp30 (AZ20) in the absence or presence of increasing concentrations of NKp46-Fc or NKp30-Fc fusion proteins (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cells were pretreated with rabbit serum (30 μL) to block Fc receptors, and human IgG1 (2 μg) was used as a negative control. Cells were incubated with NKp30-Fc or NKp46-Fc (3 μg) for 45 minutes at 4°C followed, after washing, by PE-conjugated antihuman Ig (Jackson ImmunoResearch Laboratories). 4′,6-Diamidino-2-phenylindole (Sigma-Aldrich) was added to exclude dead cells from the analysis.
HCMV preparation, sequencing of UL40 gene, and infection of moDCs
Stocks of the TB40/E HCMV strain32 (kindly provided by Christian Sinzger, Institute for Medical Virology, University of Tübingen, Tübingen, Germany) and the clinical isolate UL127133 were prepared as described previously.30 Purified stocks were resuspended in serum-free Dulbecco minimal essential medium, stored at −80°C, and titrated by standard plaque assays on MRC-5 cells. Inactivation of viral stocks was achieved by ultraviolet light using a UV-crosslinker (Bio-Rad GS genelinker UV chamber).
The sequence of the UL40 gene in HCMV isolates TB40/E and UL1271 was analyzed by direct sequencing of agarose-purified polymerase chain reaction products, which were amplified from HCMV virions using primers: 5′-TCC TCC CTG GTA CCC GAT AAC AG-3′ and 5′-CGG GCC AGG ACT TTT TAA TGG CC-3′.34
moDCs were incubated overnight alone (mock), with TB40/E (multiplicity of infection [MOI], 50-100), or with the same concentration of UV-inactivated virus. Thereafter, cells were washed twice, counted, and resuspended in RPMI 10% fetal calf serum. At 48 hours after infection, moDCs were harvested, washed, and cytospin preparations were stained by indirect immunofluorescence with a mouse anti-CMV IE-1/IE-2 monoclonal antibody (clone mab810, Chemicon) followed by FITC-conjugated goat antimouse Ig and examined as previously described.30 Briefly, slides were examined with a Leica DM6000B fluorescence microscope (HC PL fluotar 10×/0.30, dry) in Dako fluorescence mounting medium and images were taken with a Leica DFC300 FX digital camera and were analyzed with the Leica FW4000 Fluorescence Workstation software.
NK-cell functional assays
Purified fresh or IL-2-activated NK cells were resuspended in complete medium and cocultured for 24 or 48 hours with autologous moDCs (uninfected, TB40/E-infected, or treated with UV-inactivated virus) in 96-well flat-bottom plates at different moDCs/NK ratios. Surface expression of CD69 and CD25 on CD56+ cells was analyzed by flow cytometry. Culture supernatants were harvested at 24 and 48 hours, and IFN-γ concentration was measured by enzyme-linked immunosorbent assay (ELISA; Human IFN-γ Module Set; Bender MedSystems), as recommended by the manufacturer; all experiments were performed in triplicate.
Activation of NK-cell cytotoxic function was tested using the CD107a mobilization assay. PBMCs were stimulated either overnight or for 7 days with IL-2, and NK cells were subsequently purified by negative selection. Next, NK cells were incubated for 5 hours at 37°C in the presence of monensin (5 ng/mL, Sigma-Aldrich), anti-CD107a FITC (BD Biosciences PharMingen) together with autologous moDCs uninfected (mock), TB40/E-infected, or treated with UV-inactivated TB40/E; assays were performed at 48 hours or, when specified, at 72 hours after DC exposure to the virus. The HLA class I-defective erythroleukemia K562 cell line was used as a positive control for degranulation. Cells were then washed in PBS supplemented with 2mM EDTA, stained for 30 minutes at 4°C with anti-CD56 PE, and analyzed by flow cytometry. In some experiments, NK-cell functional assays were carried in the presence of saturating concentrations of a panel of NK-cell receptor-specific mAbs.
Statistical analysis
Statistical analysis was performed by the Mann-Whitney U test, using the SPSS, Version 9.0 software. Results were considered significant at the 2-sided P level of .05.
Results
NK-cell activation against autologous HCMV-infected moDCs
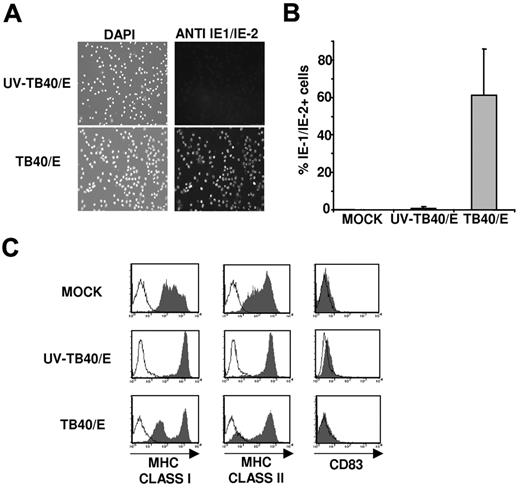
Suitable experimental conditions were established to characterize the NK-cell receptor-ligand interactions involved in the response to HCMV-infected myeloid DCs. To this end, immature moDCs were incubated with medium (mock), the TB40/E HCMV strain (MOI 50-100), or UV-inactivated TB40/E (UV-TB40/E). Cells were stained 48 hours later by indirect immunofluorescence with an mAb specific for the HCMV IE-1/IE-2 antigen (Figure 1A). Based on the percentage of IE-1/IE-2+ cells, the infection rate of moDCs varied from 40% to 90% in different experiments; nuclear IE-1/IE-2 staining was undetectable in mock and UV-TB40/E-treated cultures, although few IE-1/IE-2+ cells were occasionally stained in the latter (Figure 1B).
moDCs infected by the endothelial cell adapted TB40/E HCMV strain down-regulate major histocompatibility complex class I and II expression. Immature moDCs were incubated overnight with complete medium (MOCK), HCMV (TB40/E), or UV-inactivated HCMV (UV-TB40/E). (A) Cells were stained at 48 hours after infection with a mouse anti-CMV IE-1/IE-2 monoclonal antibody (right panels, FITC) and 4′,6-diamidino-2-phenylindole nuclear staining (left panel). Images were analyzed as described in “HCMV preparation, sequencing of UL40 gene, and infection of moDCs.” (B) Histograms represent the percentages of IE-1/IE-2+ cells detected in 8 independent experiments (mean ± SD). (C) Flow cytometry performed at 72 hours after infection. moDCs were surface labeled by indirect immunofluorescence with mAbs specific for HLA class I, class II (HLA-DR), or CD83 mAbs (open histograms represent isotype control; and filled histograms, specific staining). Results of a representative experiment (60% IE-1/IE-2+ cells) of 4 performed are shown.
moDCs infected by the endothelial cell adapted TB40/E HCMV strain down-regulate major histocompatibility complex class I and II expression. Immature moDCs were incubated overnight with complete medium (MOCK), HCMV (TB40/E), or UV-inactivated HCMV (UV-TB40/E). (A) Cells were stained at 48 hours after infection with a mouse anti-CMV IE-1/IE-2 monoclonal antibody (right panels, FITC) and 4′,6-diamidino-2-phenylindole nuclear staining (left panel). Images were analyzed as described in “HCMV preparation, sequencing of UL40 gene, and infection of moDCs.” (B) Histograms represent the percentages of IE-1/IE-2+ cells detected in 8 independent experiments (mean ± SD). (C) Flow cytometry performed at 72 hours after infection. moDCs were surface labeled by indirect immunofluorescence with mAbs specific for HLA class I, class II (HLA-DR), or CD83 mAbs (open histograms represent isotype control; and filled histograms, specific staining). Results of a representative experiment (60% IE-1/IE-2+ cells) of 4 performed are shown.
Compared with UV-TB40/E-treated moDCs, the expression of HLA class I and class II molecules decreased in HCMV-infected moDCs (Figure 1C), in agreement with previous reports.19,20 Inhibition of HLA class I expression is known to be mediated by US2, US3, US6, and US11,3 whereas the mechanisms underlying the different pattern of HLA class II down-regulation are more complex.19 The proportion of cells displaying a reduced expression of HLA class I molecules correlated with the number of IE-1/IE-2+ cells in all experiments, and time course analysis revealed that the inhibition of HLA-I expression was detectable at 48 hours (data not shown). The noninfected moDC subset in TB40/E treated cultures, as well as cells incubated with UV-inactivated TB40/E, expressed higher levels of HLA class I molecules than mock moDCs, an effect attributed to the endogenous production of type I IFN-α, according to previous reports.35 No changes in the surface levels of CD83 were observed (Figure 1C), indicating that HCMV did not induce moDC maturation, as previously described.20 The viability at 72 hours after TB40/E infection, assessed by staining with annexin V and propidium iodide, was comparable with that of control moDCs treated with UV-TB40/E, and higher than that of mock-treated cells (supplemental Figure 2); it is of note that, as described in “HCMV preparation, sequencing of UL40 gene, and infection of moDCs,” in these experiments cells were deprived of cytokines used to induce moDC differentiation.
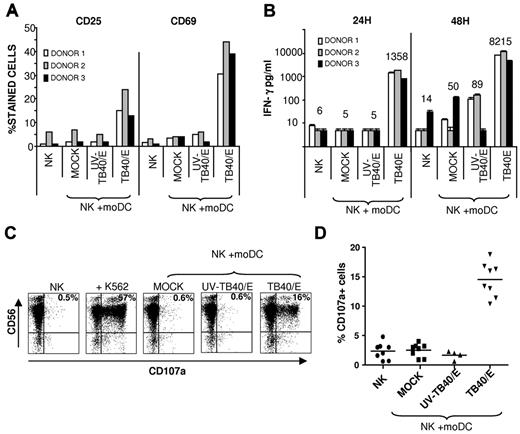
NK-cell populations from HCMV-seronegative donors were cultured alone or in the presence of autologous moDCs: untreated (mock), incubated with UV-TB40/E, or infected with TB40/E. NK cells up-regulated the surface expression of activation markers (ie, CD25 and CD69) and secreted high concentrations of IFN-γ (Figure 2A-B) in the presence of HCMV-infected moDCs, displaying a marginal or undetectable response to mock or UV-TB40/E-treated moDCs.
Specific NK-cell activation in response to autologous HCMV-infected moDCs. (A-B) Freshly purified NK cells were cultured for 48 hours alone, or with mock moDCs, UV-TB40/E-treated moDCs, or TB40/E-infected moDCs in the presence of 10 U/mL of IL-2 (moDCs/NK = 1:20). (A) CD25 and CD69 expression was analyzed by flow cytometry. (B) IFN-γ production was detected in culture supernatants by ELISA. Results from 3 representative experiments performed with different donors are shown. Numbers correspond to mean value of IFN-γ production. (C-D) NK cells purified by negative selection from PBMCs stimulated overnight with IL-2 were cocultured for 5 hours with target cells as described in “NK-cell functional assays.” Surface CD107a expression in CD56+ cells was analyzed by flow cytometry. (C) Dot plots from a representative experiment of 8 performed are shown (moDCs/NK = 1:4), including the K562 cell line as a control. The percentage of CD107a+ cells is included in each dot plot (TB40/E moDCs: 65% 1E-1/IE-2+cells) (D) Scatter plots displaying the percentage of CD107a+ cells from 8 different experiments performed.
Specific NK-cell activation in response to autologous HCMV-infected moDCs. (A-B) Freshly purified NK cells were cultured for 48 hours alone, or with mock moDCs, UV-TB40/E-treated moDCs, or TB40/E-infected moDCs in the presence of 10 U/mL of IL-2 (moDCs/NK = 1:20). (A) CD25 and CD69 expression was analyzed by flow cytometry. (B) IFN-γ production was detected in culture supernatants by ELISA. Results from 3 representative experiments performed with different donors are shown. Numbers correspond to mean value of IFN-γ production. (C-D) NK cells purified by negative selection from PBMCs stimulated overnight with IL-2 were cocultured for 5 hours with target cells as described in “NK-cell functional assays.” Surface CD107a expression in CD56+ cells was analyzed by flow cytometry. (C) Dot plots from a representative experiment of 8 performed are shown (moDCs/NK = 1:4), including the K562 cell line as a control. The percentage of CD107a+ cells is included in each dot plot (TB40/E moDCs: 65% 1E-1/IE-2+cells) (D) Scatter plots displaying the percentage of CD107a+ cells from 8 different experiments performed.
To investigate whether HCMV-infected moDCs triggered NK cell-mediated cytotoxicity, degranulation was assessed using the CD107a mobilization assay. As shown in Figure 2C-D, a significant increase in the percentage of CD107a+ CD56+ NK cells was specifically detected in response to TB40/E-infected moDCs, but not on incubation with mock or UV-TB40/E-treated moDCs. Raising the moDC/NK ratio and the MOI enhanced NK-cell activation (supplemental Figure 3).
NK-cell populations cultured for 7 days in the presence of IL-2 reacted as well preferentially against HCMV-infected moDCs by secreting IFN-γ and mobilizing CD107a. Nevertheless, in these conditions, a substantial IFN-γ production and an increase of CD107a+ cells (supplemental Figure 4A-B) were also detected in response to mock uninfected moDCs. This effect was reduced when IL-2-activated NK cells were incubated with moDCs treated with UV-TB40/E, which up-regulated HLA class I expression (Figure 1C). These results are in agreement with the reported ability of activated NK cells to react with immature moDCs and consistent with the protective role of HLA class I molecules in mature moDCs.36 As previously reported,23,25 NK-cell degranulation in response to mock moDCs was partially inhibited in the presence of mAbs specific for the NKp30 NCR and DNAM-1 but not by an anti NKp46 mAb (supplemental Figure 4C).
Studies in murine cytomegalovirus infection have revealed the important regulatory role exerted on the NK-cell response by type I IFN and IL-12, secreted in response to the virus challenge.37 In our experimental system, neutralizing IL-12 markedly inhibited IFN-γ secretion (Figure 3A), whereas blocking type I interferon receptor (IFNAR) partially reduced CD69 expression on the surface of NK cells (Figure 3B), both measured 24 hours after coculture of NK cells with moDCs. By contrast, NK cell-mediated cytotoxicity triggered by HCMV-infected moDCs at 5 hours was not significantly affected (Figure 3C). The original studies described in this section were essential to establish reliable experimental conditions required to dissect the role played by NKR-ligand interactions with infected moDCs. In that way, an overlapping response against noninfected moDCs was avoided, allowing in short-term assays to discriminate NKR involvement from the effects of cytokines.
IL-12 and IFN-α contribute to NK-cell activation induced by HCMV-infected moDCs. Freshly purified NK cells were cultured with target cells for 24 hours as described in Figure 2A-B (moDCs/NK = 1:4; TB40/E moDCs: 80% IE-1/IE-2+ cells). In parallel, the effect of IL-12-specific, IFNAR-specific, and control (myc) mAbs was tested. (A) IFN-γ production was measured by ELISA (mean ± SD of triplicates). (B) CD69 expression on NK cells was assessed by flow cytometry. NK cells purified by negative selection from PBMCs stimulated overnight with IL-2 were cocultured for 5 hours with target cells as described in “NK-cell functional assays.” In parallel, the effect of IL-12-specific, IFNAR-specific, and control (myc) mAbs was tested. Surface CD107a expression in CD56+ cells was analyzed by flow cytometry. (C) The percentage of CD56+CD107a+ is included in each dot plot. Results of a representative experiment of 3 performed are shown.
IL-12 and IFN-α contribute to NK-cell activation induced by HCMV-infected moDCs. Freshly purified NK cells were cultured with target cells for 24 hours as described in Figure 2A-B (moDCs/NK = 1:4; TB40/E moDCs: 80% IE-1/IE-2+ cells). In parallel, the effect of IL-12-specific, IFNAR-specific, and control (myc) mAbs was tested. (A) IFN-γ production was measured by ELISA (mean ± SD of triplicates). (B) CD69 expression on NK cells was assessed by flow cytometry. NK cells purified by negative selection from PBMCs stimulated overnight with IL-2 were cocultured for 5 hours with target cells as described in “NK-cell functional assays.” In parallel, the effect of IL-12-specific, IFNAR-specific, and control (myc) mAbs was tested. Surface CD107a expression in CD56+ cells was analyzed by flow cytometry. (C) The percentage of CD56+CD107a+ is included in each dot plot. Results of a representative experiment of 3 performed are shown.
Role of activating receptors in the NK-cell response to HCMV-infected moDCs
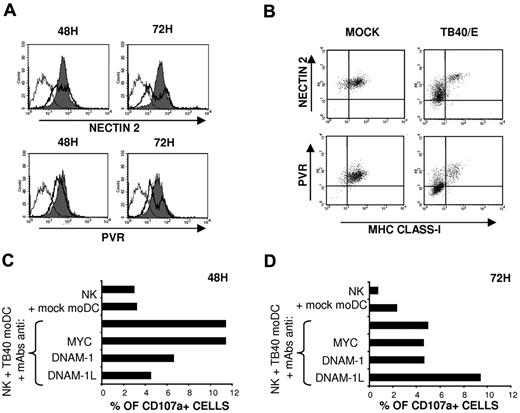
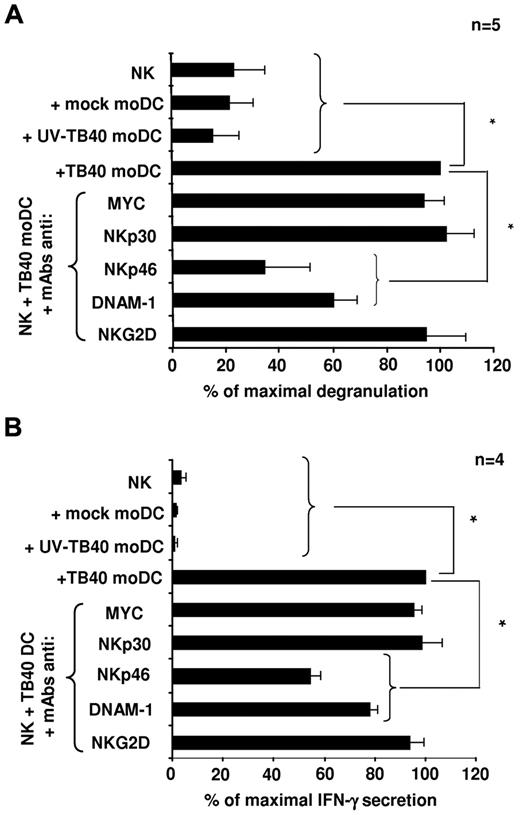
To elucidate the nature of activating receptors involved in the response to HCMV-infected moDCs, the antagonistic effect of NKR and NCR-specific mAbs (ie, anti-NKp46, NKp30, DNAM-1, NKG2D) was assessed in NK-cell degranulation assays (Figure 4A) and on the production of IFN-γ, analyzed in culture supernatants 6 hours after incubation with infected moDCs (Figure 4B). NKp30 and NKG2D-specific mAbs did not alter CD107a NK-cell expression and IFN-γ secretion, which were significantly inhibited in the presence of anti-NKp46 and DNAM-1 mAbs, thus indirectly supporting that both receptors participate in the NK-cell response against HCMV-infected moDCs (Figure 4A-B).
NK-cell degranulation and IFN-γ secretion in response to TB40/E-infected moDCs involves NKp46 and DNAM-1 activating receptors. (A) NK-cell degranulation against moDCs (moDCs/NK = 1:4) was measured by the CD107a mobilization assay in the presence of blocking mAbs as described in “NK-cell functional assays.” Assays were performed 48 hours after DC exposure to the virus. For each experiment, data were normalized to the response of NK cells incubated with HCMV-infected moDCs in the absence of mAbs (100%); in these conditions, the numbers of CD107a+ cells ranged from 11.4% to 18.8%. (B) The same experimental conditions used for degranulation assays were applied. At 6 hours, supernatants were harvested and assayed for the presence of IFN-γ by ELISA. Data were normalized to the IFN-γ levels detected in supernatants of NK cells incubated with TB40/E moDCs in the absence of mAbs (100%); in these conditions, the absolute concentrations of IFN-γ ranged from 164 to 915 pg/mL. Data are mean plus or minus SEM. *P < .05.
NK-cell degranulation and IFN-γ secretion in response to TB40/E-infected moDCs involves NKp46 and DNAM-1 activating receptors. (A) NK-cell degranulation against moDCs (moDCs/NK = 1:4) was measured by the CD107a mobilization assay in the presence of blocking mAbs as described in “NK-cell functional assays.” Assays were performed 48 hours after DC exposure to the virus. For each experiment, data were normalized to the response of NK cells incubated with HCMV-infected moDCs in the absence of mAbs (100%); in these conditions, the numbers of CD107a+ cells ranged from 11.4% to 18.8%. (B) The same experimental conditions used for degranulation assays were applied. At 6 hours, supernatants were harvested and assayed for the presence of IFN-γ by ELISA. Data were normalized to the IFN-γ levels detected in supernatants of NK cells incubated with TB40/E moDCs in the absence of mAbs (100%); in these conditions, the absolute concentrations of IFN-γ ranged from 164 to 915 pg/mL. Data are mean plus or minus SEM. *P < .05.
Expression of different NKG2D ligands (NKG2DL; ie MICA/B and ULPB1–4) was low or undetectable in uninfected moDCs and, remarkably, was not up-regulated on HCMV infection at 48 and 72 hours after infection (supplemental Figure 5; data not shown). In the case of ULBP1, a significant decrease in the expression was detected compared with control moDCs. Altogether, the data are consistent with the efficient ability of HCMV to interfere with the surface expression of NKG2DL, thus explaining the lack of antagonistic effect of anti-NKG2D mAb.
NK-cell degranulation and, to a lesser extent, IFN-γ secretion were significantly decreased in the presence of anti-DNAM-1 mAbs (Figure 4). DNAM-1 participates in NK-cell-mediated recognition of immature moDCs, which express both DNAM-1 ligands (DNAM-1L), PVR and Nectin-2.25 The UL141 HCMV protein has been reported to inhibit PVR9 and Nectin-238 expression in infected fibroblasts, contributing to immune evasion; thus, we analyzed the expression of DNAM-1L in HCMV-infected moDCs. Time-course analysis revealed that, at 48 hours after infection, the expression of both DNAM-1L on moDCs was minimally altered compared with a marked down-regulation detected at 72 hours in infected cells (Figure 5A), identified by the loss of HLA class I expression (Figure 5B). To assess the impact of the inhibition of DNAM-1L expression on the NK-cell response, the antagonistic effects of DNAM-1 or a combination of PVR and Nectin-2 specific mAbs on the response to TB40/E-infected moDCs were compared at different time points after infection. As shown in Figure 5C, HCMV-infected moDCs triggered NK-cell-mediated cytotoxicity, and degranulation was inhibited in the presence of blocking mAbs at 48 hours. By contrast, at 72 hours after infection, a reduction in the percentages of CD107a+ cells was observed, remaining unaltered in the presence of the DNAM-1 specific mAb, whereas, paradoxically, anti-DNAM-1L mAbs enhanced degranulation (Figure 5D). The data indicate that the DNAM-1 receptor plays a relevant role in the NK-cell response at early stages of HCMV infection, whereas the effects of HCMV-dependent down-regulation of DNAM-1L are perceived at later stages, thus stressing the importance of the kinetics of expression of immune evasion mechanisms.
HCMV infection down-regulates PVR and Nectin-2 expression in moDCs: influence on the NK-cell mediated response at different after infection stages. (A) Mock moDCs (filled histograms) and TB40/E moDCs (bold line, open histograms) were surface labeled at 48 hours and 72 hours after infection by indirect immunofluorescence with Nectin-2 and PVR-specific mAbs. Staining with isotype control is included (thin line, open histograms). Results of a representative experiment (65% IE-1/IE-2+ cells) of 3 performed are shown. (B) Mock moDCs and TB40/E moDCs at 72 hours after infection were also costained with FITC-conjugated HLA class I specific mAbs. Results of a representative experiment (70% IE-1/IE2+ cells) of 3 performed are shown. (C-D) NK-cell degranulation against moDCs was measured by the CD107a mobilization assay in the presence of blocking mAbs as described in “NK-cell functional assays.” Assays were performed at 48 hours (C) and 72 hours (D) after DC exposure to the virus. Results of a representative experiment of 3 performed are shown for each condition.
HCMV infection down-regulates PVR and Nectin-2 expression in moDCs: influence on the NK-cell mediated response at different after infection stages. (A) Mock moDCs (filled histograms) and TB40/E moDCs (bold line, open histograms) were surface labeled at 48 hours and 72 hours after infection by indirect immunofluorescence with Nectin-2 and PVR-specific mAbs. Staining with isotype control is included (thin line, open histograms). Results of a representative experiment (65% IE-1/IE-2+ cells) of 3 performed are shown. (B) Mock moDCs and TB40/E moDCs at 72 hours after infection were also costained with FITC-conjugated HLA class I specific mAbs. Results of a representative experiment (70% IE-1/IE2+ cells) of 3 performed are shown. (C-D) NK-cell degranulation against moDCs was measured by the CD107a mobilization assay in the presence of blocking mAbs as described in “NK-cell functional assays.” Assays were performed at 48 hours (C) and 72 hours (D) after DC exposure to the virus. Results of a representative experiment of 3 performed are shown for each condition.
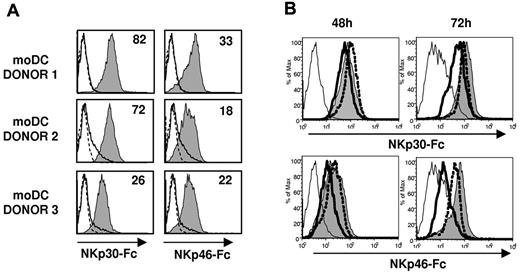
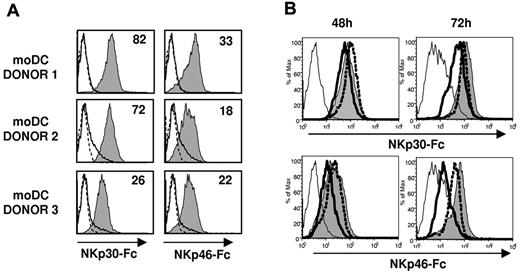
Based on the antagonistic effect of NCR-specific mAbs (Figure 4) NKp46, but not NKp30, appeared involved in the recognition of TB40/E-infected moDCs. To assess the expression of NKp30 and NKp46 ligands in moDCs, we analyzed by flow cytometry the binding of NCR-Fc fusion proteins. As shown in Figure 6A, both NKp30-Fc and NKp46-Fc clearly stained moDCs, thus providing, to our knowledge, the first evidence that these cells constitutively express surface ligands for both NCRs. At 48 hours after infection, when functional assays were performed, staining of infected moDCs by soluble NCR was partially reduced. By contrast, a marked decrease of NKp30-Fc and, especially of NKp46-Fc specific binding to moDCs, was observed at 72 hours after infection (Figure 6B). It is of note that, compared with the selective loss of HLA class I and DNAM-1L in infected cells (bimodal distributions in Figures 1C, 5A), down-regulation of NCR ligands homogeneously affected all TB40/E-treated moDCs (Figure 6B), including both infected and noninfected cells.
NKp30 and NKp46 ligands are constitutively expressed on moDCs and are down-regulated at late stages of HCMV infection. (A) NKp30 and NKp46 ligand expression on moDCs was assessed by indirect immunofluorescence and flow cytometry using soluble recombinant NKp30-Fc and NKp46-Fc fusion proteins as described in “Expression of NCR-Fc chimeric constructs and immunofluorescence.” Staining with NKp30-Fc and NKp46-Fc (filled histograms) was compared with control human IgG1 (thin line, open histograms) and with secondary antibodies alone (dotted line, open histograms). Inserted numbers correspond to geometric means. (B) Mock moDCs (filled histograms), UV-TB40/E moDCs (dotted line, open histograms), and TB40/E moDCs (bold line, open histograms) were surface labeled at 48 hours and 72 hours after virus exposure by indirect immunofluorescence with NKp30-Fc and NKp46-Fc. Staining with a human IgG1 is included as a control (thin open histograms). Results of a representative experiment (70% IE-1/IE-2+ cells) of 4 performed are shown.
NKp30 and NKp46 ligands are constitutively expressed on moDCs and are down-regulated at late stages of HCMV infection. (A) NKp30 and NKp46 ligand expression on moDCs was assessed by indirect immunofluorescence and flow cytometry using soluble recombinant NKp30-Fc and NKp46-Fc fusion proteins as described in “Expression of NCR-Fc chimeric constructs and immunofluorescence.” Staining with NKp30-Fc and NKp46-Fc (filled histograms) was compared with control human IgG1 (thin line, open histograms) and with secondary antibodies alone (dotted line, open histograms). Inserted numbers correspond to geometric means. (B) Mock moDCs (filled histograms), UV-TB40/E moDCs (dotted line, open histograms), and TB40/E moDCs (bold line, open histograms) were surface labeled at 48 hours and 72 hours after virus exposure by indirect immunofluorescence with NKp30-Fc and NKp46-Fc. Staining with a human IgG1 is included as a control (thin open histograms). Results of a representative experiment (70% IE-1/IE-2+ cells) of 4 performed are shown.
CD94/NKG2A+ NK cells efficiently respond to HCMV-infected moDCs, which down-regulate HLA-E expression
A leader signal peptide from the UL-40 HCMV protein has been reported to stabilize the surface expression of HLA-E in fibroblasts, thus repressing NK cells bearing the CD94/NKG2A inhibitory receptor.12,13 On the other hand, indirect evidence has been obtained supporting that CD94/NKG2C+ NK cells may be involved in the response to HCMV.15,16
Thus, we comparatively assessed the response of CD94/NKG2A+ and CD94/NKG2C+ NK cells from selected HCMV+ blood donors against HCMV-infected moDCs using the CD107 mobilization assay. Remarkably, CD94/NKG2A+ NK cells degranulated against HCMV-infected immature moDCs more efficiently than the NKG2C+ subset, whereas both comparably reacted against the HLA class I-negative K562 leukemia cell line (Figure 7A).
HCMV-infected moDCs trigger a preferential response of CD94/NKG2A+ NK cells associated with a down-regulation of HLA-E expression. (A) NK cells purified by negative selection from PBMCs stimulated overnight with IL-2 were cocultured for 5 hours with target cells as described in “NK-cell functional assays.” Surface CD107a expression on CD94/NKG2A+ and CD94/NKG2C+ NK cells was analyzed by flow cytometry. Dot plots from 2 representative donors of 6 analyzed are shown. The proportions of CD107a+ cells referred to total NKG2A+ or NKG2C+ cells are specified in bold. (B) moDCs were surface labeled 48 hours after virus exposure by indirect immunofluorescence with an HLA-E-specific mAb (3D12) (open histograms, isotype control; filled histograms specific staining). Fluorescence intensity (geometric mean) of selected populations is included. Results of a representative experiment (60% IE-1/IE-2+ cells) of 6 performed are shown. (C) Alignment of part of gpUL40 amino acid sequences from AD169, TB40/E, and the HCMV clinical isolate UL1271; predicted leader sequences are shaded, and HLA-E binding peptides are boxed. (D) moDCs were mock treated (filled histograms) or infected with the HCMV clinical isolate UL1271 (MOI 25: gray line; MOI 100: black line, open histograms) and surface labeled at 48 hours and 72 hours after virus exposure by indirect immunofluorescence with mAbs specific for HLA-E (3D12) and HLA class I molecules (HP-1F7). Results of a representative experiment (MOI 25: 25% IE-1/IE-2+ cells; MOI 100: 85% IE-1/IE-2+ cells) of 3 performed are shown.
HCMV-infected moDCs trigger a preferential response of CD94/NKG2A+ NK cells associated with a down-regulation of HLA-E expression. (A) NK cells purified by negative selection from PBMCs stimulated overnight with IL-2 were cocultured for 5 hours with target cells as described in “NK-cell functional assays.” Surface CD107a expression on CD94/NKG2A+ and CD94/NKG2C+ NK cells was analyzed by flow cytometry. Dot plots from 2 representative donors of 6 analyzed are shown. The proportions of CD107a+ cells referred to total NKG2A+ or NKG2C+ cells are specified in bold. (B) moDCs were surface labeled 48 hours after virus exposure by indirect immunofluorescence with an HLA-E-specific mAb (3D12) (open histograms, isotype control; filled histograms specific staining). Fluorescence intensity (geometric mean) of selected populations is included. Results of a representative experiment (60% IE-1/IE-2+ cells) of 6 performed are shown. (C) Alignment of part of gpUL40 amino acid sequences from AD169, TB40/E, and the HCMV clinical isolate UL1271; predicted leader sequences are shaded, and HLA-E binding peptides are boxed. (D) moDCs were mock treated (filled histograms) or infected with the HCMV clinical isolate UL1271 (MOI 25: gray line; MOI 100: black line, open histograms) and surface labeled at 48 hours and 72 hours after virus exposure by indirect immunofluorescence with mAbs specific for HLA-E (3D12) and HLA class I molecules (HP-1F7). Results of a representative experiment (MOI 25: 25% IE-1/IE-2+ cells; MOI 100: 85% IE-1/IE-2+ cells) of 3 performed are shown.
A reduced surface expression of HLA-E was detected in TB40/E-infected moDCs, thus providing an explanation for these unexpected observations (Figure 7B). By contrast, the noninfected moDC subset up-regulated HLA-E in parallel to total HLA class I, comparable to cells treated with UV-inactivated TB40/E, consistent with their response to endogenous type I IFN as discussed for Figure 1. An obvious question was whether the UL40 leader signal peptide from TB40/E displayed the canonical sequence reported to stabilize HLA-E (VMAPRTLIL). Although this issue was not addressed in a detailed report on TB40/E,39 the annotated sequence (GenBank accession number EF999921) included a mutation in p2, a key anchor residue, where Met was substituted by Val; sequencing our TB40/E batch confirmed the mutation. Thus, we considered to what extent down-regulation of HLA-E expression in TB40/E-infected moDCs might be attributed to this structural change. The possibility to generate a revertant using the conventional approach based on the use of a TB40/E BAC was ruled out, as this mutant lacks the US2, US3, and US6 genes, thus preserving in infected cells high surface levels of all HLA class I molecules, including HLA-E (data not shown). To circumvent this drawback, we tested a different HCMV clinical isolate (UL1271)33 capable of infecting moDCs and bearing the UL40 peptide canonical sequence (Figure 7C). HLA-E was also clearly down-regulated in moDCs infected with this HCMV clinical isolate (Figure 7D), compared with its expression levels in mock moDCs. On the other hand, similar to TB40/E, UL1271 treatment also enhanced total HLA class I and HLA-E expression in the residual noninfected moDC subset (Figure 7D). These observations point out that UL40 is inefficient to protect infected moDCs against NKG2A+ NK cells, in contrast to its role in immune evasion reported in fibroblasts.
Discussion
A vigorous specific NK-cell response to autologous HCMV-infected moDCs was detected by the expression of activation markers, IFN-γ production, and NK-cell degranulation. The marginal response to mock-infected or UV-TB40/E-treated moDCs supported that NK-cell activation was triggered by the fraction of infected cells in which HLA class I molecules were down-regulated. In line with previous studies in influenza-infected moDCs,40 type I IFN and IL-12 contributed to the response against HCMV-infected cells, complementing the NKR/NCR-dependent signals. In this regard, our results revealed a dominant role of NKp46 and DNAM-1 in NK-cell recognition of HCMV-infected moDCs, without a detectable involvement of NKp30 and NKG2D. These observations pointed out clear differences with NK-cell response to noninfected moDCs, where anti-DNAM-1 and NKp30, but not anti NKp46 mAbs, inhibited NK activation.
NKG2D has been reported to be involved in the response to murine cytomegalovirus-infected DCs37 and is thought to participate as well in the defense against HCMV.41 In this case, the evidence is essentially indirect and based on the identification of viral immune evasion molecules in HCMV (ie, UL16, UL142, and miR–UL112)4-7 that selectively interfere with the surface expression of NKG2D ligands (NKG2DL), similar to those used by murine cytomegalovirus (m138, m145, m152, and m155).8 The existence of multiple CMV strategies targeting the expression of NKG2DL in different species is interpreted as evidence for the strong evolutionary pressure exerted by the lectin-like receptor in antiviral defense. The expression of NKG2DL appeared virtually undetectable in TB40/E-infected moDCs, reflecting the effectiveness of immune evasion mechanisms and consistent with the lack of antagonistic effects of anti-NKG2D mAbs in the response of NK cells. In contrast, NKG2DL were shown to be up-regulated in influenza-infected moDCs, contributing to trigger NK-cell activation.40
DNAM-1 was reported to participate in the response of activated NK cells against autologous immature moDCs, which express PVR and Nectin-2.25 Functional studies also supported the importance of this receptor-ligand system in the NK-cell response to HCMV-infected moDCs. The UL141 HCMV molecule was originally reported to inhibit the surface expression of PVR, interfering with NK-cell-mediated recognition of infected fibroblasts.9 We confirmed that this DNAM-1 ligand was down-regulated in TB40/E-infected moDCs; and in agreement with a recent report,38 we detected a similar effect on the expression of Nectin-2. Time-course analysis indicated that inhibition of DNAM-1L was limited at 48 hours after infection, when the NK-cell response was assessed, becoming marked at 72 hours. Blocking DNAM-1 or DNAM-1L with specific mAbs comparably inhibited NK activation at 48 hours, whereas anti-DNAM-1L mAbs enhanced degranulation at 72 hours. This paradoxical effect might be explained by the involvement of TIGIT, a recently identified inhibitory receptor expressed by T and NK cells that binds PVR with higher affinity compared with DNAM-1.42,43 The partial loss of PVR and, particularly, of Nectin-2 expression in infected cells below a critical threshold might impair activation via DNAM-1 while maintaining TIGIT-dependent negative signaling; further studies are required to directly verify this hypothesis. Thus, DNAM-1 plays a relevant role in NK-cell recognition of HCMV-infected moDCs early during infection, whereas the effect of viral-mediated down-regulation of DNAM-1L prevails at later stages, thus illustrating the importance of the kinetics of immune evasion mechanisms.
Based on the antagonistic effect of NCR-specific mAbs, the response to HCMV-infected moDCs was dependent on NKp46, in contrast to the dominant role played by NKp30 in the response of IL-2-activated NK cells to immature noninfected moDCs.23,24 NKp46 was originally reported to interact with influenza hemagglutinin,31 contributing with NKG2D to trigger the NK-cell response against influenza-infected moDCs.40 By contrast, no HCMV molecules interacting with this NCR have been identified, and the nature of its cellular ligands remains unknown. It is of note that the molecular basis for NKp30-mediated recognition of noninfected moDCs is also uncertain, as they do not display the B7-H6 ligand.44
Taking advantage of the availability of soluble NCR-Fc fusion proteins, we observed that both NKp30-Fc and NKp46-Fc specifically bound to the surface of noninfected moDCs. To our knowledge, this provides the first unequivocal evidence that ligands for both NCR are constitutively expressed by this cell type. Time-course analysis during HCMV infection revealed that binding of NCR fusion proteins did not increase at 48 hours after infection, when the NK-cell response was assayed but appeared reduced at later stages. Down-regulation of NCR ligands became more evident for NKp46 and, moreover, had an impact on the NK-cell response that was not anymore antagonized by anti NKp46 mAbs at 72 hours (data not shown). Hence, the dominant role of NKp46 played in the response could not be simply explained by an induction of NCR ligand expression in HCMV-infected moDCs. It is conceivable that NKp46 may trigger NK activation simply as a result of the down-regulation of HLA class I expression in infected cells resulting in the loss of inhibitory NKR signaling, in agreement with the missing self-hypothesis, as proposed.45 Alternatively, the possibility that qualitative changes in the conformation/structure of NKp46 ligand might take place during infection, increasing the affinity for the receptor, cannot be ruled out.
On the other hand, in line with previous functional studies,22-24 our data support the expression of an NKp30L in moDCs, different from the B7H6 molecule. Binding of NKp30-Fc to infected moDCs remained essentially unaltered at 48 hours, and the basis for the apparent lack of involvement of NKp30 in the response to HCMV-infected moDCs is uncertain. By contrast, NKp30 participated in the response of 7-day IL-2-activated NK cells, but not of freshly isolated NK cells, against noninfected moDCs, which display NKp30 ligand(s) and normal levels of HLA class I molecules. The data suggest that the function of this NCR may depend on the metabolic status of the NK cell. In this regard, NKp30 surface expression levels were up-regulated after 7-day stimulation with IL-2 (data not shown). On the other hand, the pp65 (UL83) tegument HCMV protein was reported to interact with NKp30 and to interfere with signaling, apparently uncoupling the receptor from its adaptor molecule by a still undefined mechanism.46 Thus, the possibility that pp65 released by infected cells might selectively impair NKp30-mediated activation should be also considered.
Further studies are required to characterize the molecular nature of the NKp30 and NKp46 ligands expressed by moDCs that were decreased at late stages after infection (72 hours). It is of note that the loss of NCR ligand expression homogeneously affected all cells in TB40/E-treated cultures, including the noninfected cell fraction (IE1/2-negative) where class I expression was preserved. The mechanism underlying this effect, and in particular the putative role played by soluble factors produced by HCMV-infected cells, is currently being investigated.
NKG2A+ NK cells degranulated more efficiently than the NKG2C+ subset in response to infected moDCs, in which surface HLA-E expression was down-regulated. A mutation in p2 (Met/Val) of the TB40/E UL40 nonamer binding to HLA-E was detected but did not account for its inability to preserve the class Ib molecule levels in HCMV-infected moDCs, which was confirmed on infection with a clinical isolate bearing the canonical UL40 signal peptide sequence (VMAPRTLIL). Together, the data support that the ability of UL40 to stabilize HLA-E expression, as originally described in fibroblasts,13 is inefficient to preserve the class Ib molecule surface levels in moDCs and to prevent NKG2A+ NK-cell activation. Further studies are required to evaluate the impact that the UL40 mutation may have on HLA-E expression by HCMV-infected fibroblasts, as well as on the response of NK cells and of the cytotoxic T lymphocyte subset reported to specifically recognize the class Ib molecule through the TcR47,48 ; eventually, this might allow to understand the basis for the selection of the mutation in TB40/E.
Compared with NKG2A+ cells, the NKG2C+ NK-cell subset includes higher proportions of KIR+ and LILRB1+ cells and bears lower surface levels of NKp30 and NKp46 NCR.14 This might explain the lower response of CD94/NKG2C+ cells against HCMV-infected moDCs, also reported in fibroblasts,49 that appears contradictory with their putative involvement in the response to the virus. Although the mechanism(s) underlying the late expansion of the NKG2C+ NK-cell subset in response to HCMV infection15 remain unknown, the phenomenon is reminiscent of the expansion of circulating virus-specific specific cytotoxic T lymphocyte displaying a terminally differentiated phenotype, often associated with NKR expression, which exhibit reduced effector functions against virus-infected cells.50
In conclusion, our results support that human NK cells are capable of effectively counteracting viral immune evasion strategies and responding to infected moDCs that have impaired their antigen-presenting functions, thus indirectly favoring the development of adaptive immune responses to viral antigens cross-presented by healthy DCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Esther Menoyo for her support in acquiring blood samples, Gemma Heredia for excellent technical assistance, Dr Oscar Fornas for advice in flow cytometry, the blood donors, and especially Dr Ofer Mandelboim for generously supplying the plasmids for expression of NKp30-Fc and NKp46-Fc and Dr Christian Sinzger for kindly providing TB40/E.
This work was supported by Ministerio de Ciencia e Innovación (SAF2007-61 814), Marie Curie Training Network, European Union (MRTN-CT-2005-019284), Instituto de Salud Carlos III (Red HERACLES; M.L.-B.) and Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità (agreement 40G.41), Ministero della Salute (Ricerca Finalizzata 2005/agreement 57 and Ricerca Oncologica-Project of Integrated Program 2006-08 agreements RO strategici 3/07 and Progetto di Ricerca di Ateneo 2008; A. Moretta). G.M. is supported by MRTN-CT-2005–019284. A. Muntasell is recipient of a fellowship from the Juan de la Cierva Program. N.R. was supported by a fellowship from Instituto de Salud Carlos III. A.S.-B. was supported by a fellowship from Departament d'Universitats, Recerca i Societat de la Informació (Generalitat de Catalunya).
Authorship
Contribution: G.M. designed and performed experiments, analyzed results, and wrote the manuscript; M.L.-B. designed the research, analyzed results, and wrote the manuscript; A. Muntasell and A.A. analyzed and discussed results; N.R. and A.S.-B. helped in the experimental work; and D.P., A. Moretta, D.E.G., and H.H. provided essential reagents and scientific advice.
Conflict-of-interest disclosure: A. Moretta is founder and shareholder of Innate-Pharma. The remaining authors declare no competing financial interests.
Correspondence: Miguel López-Botet, Universitat Pompeu Fabra, Doctor Aiguader 88, 08003 Barcelona, Spain; e-mail: miguel.lopez-botet@upf.edu.