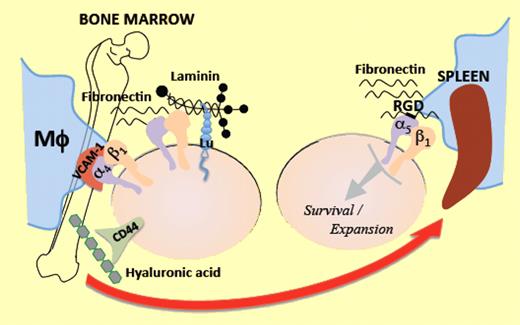
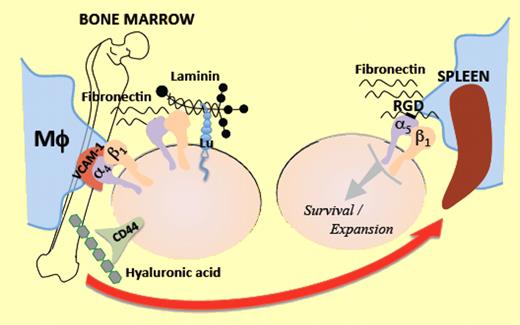
In this issue of Blood, Ulyanova and colleagues present a systematic analysis of the role of β1 integrins in erythropoiesis and demonstrate that α5β1 integrin is a critical requirement for mounting successful stress erythropoiesis.1
Stress erythropoiesis is increasingly recognized as more than simply an expansion of steady-state erythropoiesis. Rather, it is a process arising from unique progenitor cells with distinct quantitative and qualitative cytokine requirements. Increased erythropoietin (Epo) levels resulting from kidney hypoxia due to anemia, initiate the response of stress erythropoiesis. A progenitor, named stress burst-forming unit-erythroid (BFU-E) and found in the spleen but not in bone marrow of mice subjected to erythropoietic stress, has been identified as able to produce erythroid colonies when exposed to high Epo concentrations with no additional in vitro requirement for stem cell factor (SCF) or interleukin-3, in contrast to bone marrow BFU-E.2 Stress BFU-E are stimulated to expand by bone morphogenetic protein 4 (BMP4) produced in the spleen in response to hypoxia. Erythropoietic stress leads to mobilization of bone marrow progenitors which home to the spleen and differentiate into stress BFU-E, under the influence of BMP4 and Hedgehog signaling.3 Nevertheless, homeostatic erythropoietic pathways like SCF–c-kit and glucocorticoid receptor–mediated signaling are also vital for successful stress erythropoietic response in vivo.4,5 Stress erythropoiesis has been shown to be impaired in gene-targeted mouse models where steady-state erythropoiesis is fairly normal, like in mice with deficiency of growth arrest–specific gene 6 (Gas6)6 or conditional deletion of focal adhesion kinase (FAK).7 On the other hand, stress erythropoiesis in the splenic microenvironment can be successful in cases where homeostatic erythropoiesis in the bone marrow is pathologic, like in deficiency of Rac1 and Rac2 GTPases.8
The interactions of erythroid progenitors and precursors with macrophages and extracellular matrix components such as fibronectin and laminin within erythroblastic islands are critical for optimal proliferation, survival, differentiation, and terminal maturation into red blood cells.9 α4β1 and α5β1 integrins are the main erythroid cell receptors that interact with fibronectin. Whereas α4β1 mediates adhesion to several sites on fibronectin, α5β1 binds predominantly to the Arg-Gly-Asp (RGD) domain. α5 expression is noted to be higher in early erythroblasts, whereas α4 is widely expressed throughout nucleated erythroid cells. VCAM-1 represents the major and preferred ligand for α4β1 in stroma cells. The role of these integrins and their ligands in erythropoiesis has been extensively studied by in vitro and in vivo assays, frequently with contradictory results. In this issue, Ulyanova and colleagues offer a resolution to these conflicts, using a systematic in vivo genetic approach to analyze the erythropoietic phenotype after conditional deletion of β1, α4, or VCAM-1 in hematopoietic tissues of adult mice.1 β1 deletion produces β1Δ/Δ mice which demonstrate deficiency of α5β1 and compensatory overexpression of α4β7 in erythroid cells. α4Δ/Δ mice are deficient in α4β1 and α4β7 (but not α5β1) in hematopoietic cells. β1Δ/Δ, α4Δ/Δ, and VCAM-1Δ/Δ mice have normal homeostatic erythropoiesis. Ulyanova and colleagues proceed to examine their stress erythropoiesis response to phenylhydrazine (PHZ). The survival of β1Δ/Δ mice is severely compromised due to their inability to mount successful life-saving splenic stress erythropoiesis, despite having a robust response to PHZ in the bone marrow regarding progenitor cell expansion and mobilization to peripheral blood. BFU-E, stress BFU-E, and CFU-E are all drastically diminished in β1Δ/Δ spleen, associated with increased apoptosis in splenic erythroblasts. To determine whether the phenotype observed is due to β1 integrin deficiency in erythroid or stroma cells, Ulyanova and colleagues used irradiated wild-type recipient mice transplanted with β1Δ/Δ donor cells. These mice demonstrate similar although slightly improved phenotypic response, indicating that the great majority of the effects seen in β1Δ/Δ animals are predominantly hematopoietic cell autonomous.
An additional ingenious contribution of this study is the assay used to dissociate homing/lodgment in spleen from subsequent expansion/engraftment. Wild-type mice were injected with control or β1Δ/Δ bone marrow cells. Twenty-four hours later, recipients were killed and spleen cells were plated in methylcellulose colony assays. Each colony produced was genotyped to determine the origin (host vs donor) and proportion of homed progenitors. There was no significant impairment of splenic homing of β1Δ/Δ cells. Engraftment was evaluated by CFU-S assessment, 11 days after transplantation of lethally irradiated wild-type recipients. Despite the normal homing, the development of CFU-S was significantly impaired in mice transplanted with β1-deficient hematopoietic cells suggesting impairment in survival and/or expansion of homed progenitors. α4-deficient mice demonstrate a sufficient, although delayed, mounting of stress erythropoiesis and α4Δ/Δ hematopoietic cells perform normally in the above assays, implicating that the absence of α5β1-mediated signaling within the splenic microenvironment is responsible for the decreased progenitor cell expansion and survival observed in the spleen of β1-deficient animals. Both α4Δ/Δ and β1Δ/Δ mice have terminal maturation defects in both bone marrow and spleen, pointing to the important role of α4β1 in the late steps of erythroid differentiation.10 The authors propose that retention and subsequent expansion of erythroid cells in the spleen is mainly an RGD-dependent process mediated by the α5β1 integrin, in contrast to bone marrow, where other ligands in stroma cells or matrix components offer redundant signals to erythroid cells via receptors such as CD44, Lutheran glycoprotein (Lu), α4β1, and α4β7 integrins (see figure).
Erythroid progenitors in the bone marrow interact via multiple receptors with multiple ligands on macrophages (Mϕ) and matrix components, within the erythropoietic niche. When they are mobilized under erythropoietic stress toward the spleen, the significance of α5β1 integrin to attain “engraftment,” expansion, and survival becomes prominent.
Erythroid progenitors in the bone marrow interact via multiple receptors with multiple ligands on macrophages (Mϕ) and matrix components, within the erythropoietic niche. When they are mobilized under erythropoietic stress toward the spleen, the significance of α5β1 integrin to attain “engraftment,” expansion, and survival becomes prominent.
This study presents yet another unique requirement for stress erythropoiesis in contrast to homeostatic erythropoiesis, and raises several intriguing questions for future research. Is the structure of erythroblastic island different in extramedullary versus medullary erythropoiesis? Which are the signaling pathways triggered by β1 integrins within the erythroid progenitors after interaction with ligands on the stroma cells and matrix components of the erythropoietic niche and what are the gene expression patterns they induce in combination or distinctly? Are the RGD-dependent interaction and α5β1-mediated signaling potential targets to decrease stress erythropoiesis in conditions where stress-induced extramedullary erythropoiesis may cause more harm than benefit, like in thalassemias? Answers to these questions would further our understanding of the molecular control of erythropoiesis in health and disease and direct these findings toward future clinical applications.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health