In this issue of Blood, Hulbert and colleagues prospectively look at a population of 40 patients with sickle cell disease (SCD) who were being regularly transfused for the prevention of recurrent stroke. They found an alarming incidence of stroke progression as well as development of new silent strokes. Progressive infarction occurred in almost one-half of the patients, 20% developed new overt strokes, and ∼ 27% developed new silent strokes despite aggressive transfusion therapy.
Not surprisingly, the presence of vessel narrowing on magnetic resonance angiography (MRA) strongly predicted progressive stroke.1 Although this study is not the first to report progression of cerebrovascular disease in transfused patients with SCD,2 the article raises some very interesting points that are relevant clinically and have bearing on basic predictions based on in vitro blood rheology,3 as discussed below. Importantly, Hulbert et al show that progression of sickle cerebrovascular disease can occur even in patients whose average hemoglobin S (HbS) concentrations were maintained well within the currently accepted target range for stroke prophylaxis. At a minimum, these observations bring into question the target HbS concentration required for stroke protection.
While factors like inflammation, endothelial function, coagulation, and vessel reactivity contribute to vaso-occlusion, transfusion exerts its effect almost exclusively by changing the properties of the blood itself. Why should creating a mixture of normal and sickle red cells by transfusion minimize or eliminate vaso-occlusive pathology? If all HbS-containing red cells were replaced with normal red cells, blood rheologic properties would normalize. Less than complete replacement should have intermediate effects. It is well known that blood viscosity is proportional to hematocrit; for a given hematocrit, sickle cell blood is more viscous than normal blood and unlike normal blood, deoxygenated sickle blood is more viscous than oxygenated blood. Importantly, blood is a non-Newtonian fluid, which means that its viscosity is lower when it is flowing faster. All of these properties affect the ability of transfusion to improve blood flow, particularly in the microvascular environment thought to be related to silent strokes.4
The basic “occlusive unit” is the capillary bed and associated pre- and postcapillary microvessels (see figure). Red cell deformability is the dominant factor in the capillary because the red cell must bend to enter the capillary. The flexible, oxygenated HbS-containing red cell enters the capillary and releases its oxygen, after which the deoxygenated HbS polymerizes causing the cell to become rigid.5 If the transit time through the capillary is longer than the time for HbS polymerization, the rigid red cell will become lodged in the capillary. All proximal HbS-containing red cells will become lodged as well. In a mixture of SS red cells and transfused normal red cells, a greater proportion of SS red cells will increase the probability that a red cell will become lodged and will decrease the likelihood that a branch will reopen since the proximal HbS red cells will not be able to escape before becoming rigid.
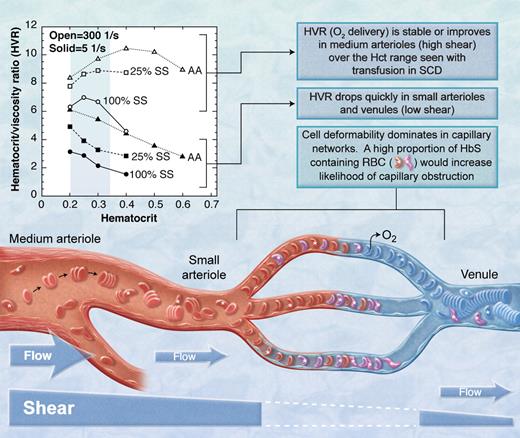
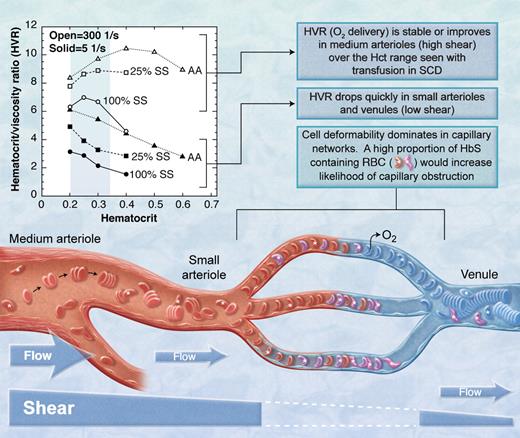
The HbS containing red blood cells (RBC, marked with *) in the mixture of sickle (SS) and normal (AA) red blood cells must transmit the microvasculature into the larger venule before they sickle or they will obstruct flow. Open symbols indicate high shear; closed symbols, low shear.3 (Professional illustration by A. Y. Chen.)
The HbS containing red blood cells (RBC, marked with *) in the mixture of sickle (SS) and normal (AA) red blood cells must transmit the microvasculature into the larger venule before they sickle or they will obstruct flow. Open symbols indicate high shear; closed symbols, low shear.3 (Professional illustration by A. Y. Chen.)
In vessels larger than capillaries, blood flow is determined in part by the bulk viscosity of blood which is affected by the viscous properties of red cells in plasma and, importantly, by hematocrit. Bulk viscosity is also a function of shear, which is a function of flow rate in a vessel. In low-shear environments such as postcapillary venules and some precapillary arterioles, discoid red cells form rouleaux (ie, stacks of red cells) that make the blood much more viscous (see figure). However, in high-shear areas such as the middle meningeal artery, rouleaux are dispersed by shear forces and blood viscosity is reduced. Adding sickle red cells to a suspension of normal cells increases the viscosity at high and low shear. With this in mind, consider the findings of Alexy and colleagues who studied the viscosity of mixtures of normal and sickle red cells in plasma at various shear levels.3 Their results are presented in the figure as the hematocrit/blood viscosity ratio (HVR) versus hematocrit. HVR is an index of the ability of blood to transport oxygen to the microvasculature. Over the range of hematocrit from 20% to 33% commonly seen in chronically transfused SCD patients, HVR at high shear improves with increasing hematocrit and then becomes stable (open symbols in the figure). However, at low shear (solid symbols in the figure), such as one might expect in pre- and postcapillary vessels, HVR decreases significantly with increasing hematocrit. Thus, flow through the low-shear pre- and postcapillary network would be expected to decrease with trans-fusion, making it more likely that HbS-containing red cells entering the capillary will not exit the capillary before they sickle and further obstruct flow.
Based upon the HVR versus hematocrit concepts presented above, transfusion would not prevent microvascular events like silent strokes. Rather, transfusion could possibly make them worse. Furthermore, HbS levels would have to be much less than the 25% achieved in the current study for the low-shear viscosity properties to approach normal. Hulbert et al report stroke at levels of HbS < 20% in some patients.1 These clinical results are consistent with the theoretical prediction based on HVR data (ie, HbS < 25% to normalize low shear viscosity): 27% of the subjects had new silent strokes in spite of average HbS levels of 26%. The observation by others that pain (presumably due to occlusion in low-shear tissues like bone and bone marrow) is more frequent in patients with higher hematocrit6 and is not relieved acutely by transfusion is also consistent with this concept. Conversely, at high shear, HVR and flow in the middle meningeal artery and similar or larger-sized vessels should be improved by transfusion. Certainly, transfusion significantly reduces stroke recurrence rates in SCD patients with larger artery disease7 and reduces transcranial Doppler velocity.
While the clinical outcome of transfusion1 is consistent with predictions made from in vitro rheology, the pathophysiology is certainly more complicated than implied herein. For example, the prediction assumes, perhaps incorrectly, that silent strokes are due to sickle-related vaso-occlusion.4 Similar silent stroke-like lesions occur in more than 50% of thalassemia intermedia patients8 and in normal subjects as well.9 Duration of exposure to high shear results in change in endothelial function10 which further complicates the picture. Current information1 does not allow us to determine the level of HbS necessary to protect against progression of cerebrovascular disease, in part because only the average HbS values but not the time-average values before events are known. However, from a practical standpoint, there is a progression of cerebrovascular disease in spite of chronic transfusion according to best practices at centers of excellence.1 The results of the silent infarct transfusion study11 will thus be of critical importance and hopefully will shed more light on these issues. Clearly, transfusion is not a panacea and more work is needed to understand how transfusion works and how to prevent the progressive vascular disease seen in sickle cell patients.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgments
The author thanks Martine Torres, PhD, and Hebert Meiselman, ScD, for review and helpful comments.