Abstract
Acute graft-versus-host disease (aGVHD) is associated with high risk of morbidity and mortality and is a common complication after double umbilical cord blood (UCB) transplantation. To reduce these risks, we established a method of CD4+CD25+FoxP3+ T regulatory cell (Treg) enrichment from cryopreserved UCB followed by a 18 + 1-day expansion culture including anti-CD3/anti-CD28 antibody-coated beads and recombinant human interleukin-2. In a “first-in-human” clinical trial, we evaluated the safety profile of UCB Treg in 23 patients. Patients received a dose of 0.1-30 × 105UCB Treg/kg after double UCB transplantation. The targeted Treg dose was achieved in 74% of cultures, with all products being suppressive in vitro (median 86% suppression at a 1:4 ratio). No infusional toxicities were observed. After infusion, UCB Treg could be detected for 14 days, with the greatest proportion of circulating CD4+CD127−FoxP3+ cells observed on day +2. Compared with identically treated 108 historical controls without Treg, there was a reduced incidence of grade II-IV aGVHD (43% vs 61%, P = .05) with no deleterious effect on risks of infection, relapse, or early mortality. These results set the stage for a definitive study of UCB Treg to determine its potency in preventing allogeneic aGVHD. This study is registered at http://www.clinicaltrials.gov as NCT00602693.
Introduction
Regulatory T cells (Tregs) represent a novel cell-based approach for potentially reducing the risk of severe acute graft-versus-host disease (aGVHD). Tregs are a subset of CD4+ T cells that coexpress CD25 (interleukin-2Rα chain) and high levels of Foxp31 and are dependent on interleukin-2.2 Our group and others have shown that in murine models lethal aGVHD can be prevented by Tregs, with enhanced survival.3-8 In these models of aGVHD, CD4+/CD25+ Treg cells functioned at least in part through the suppression of CD8+ effector cells expansion in GVHD target organs.7 In contrast, depletion of CD4+/CD25+ Treg cells increased aGVHD lethality.5 Further, Tregs inhibited the development of chronic GVHD9-11 and facilitated engraftment in murine models of allogeneic transplantation.7,12,13
Double umbilical cord blood transplantation (DUCBT) has been shown to overcome the cell-dose limitation that often prevents the use of this treatment modality in adults and larger adolescents.14-16 However, compared with single UCBT, a significantly greater risk of grade II aGVHD17 is observed after DUCBT. Regardless of the source of allogeneic hematopoietic stem cells (HSCs), severe forms of aGVHD are associated with an increased risk of morbidity and mortality.15,17
To date, there are no reports in the literature that document the safety and efficacy of ex vivo–expanded natural Tregs. Therefore, we designed a phase 1 dose-escalation trial to study the safety and feasibility of the infusion of Tregs isolated from a partially human leukocyte antigen (HLA)-matched UCB unit and ex vivo expanded in culture. We report here the result of the first-in-human clinical trial of UCB-derived CD4+/CD25+ Tregs in the setting of UCBT.
Methods
Patient inclusion criteria
Patients with advanced or high-risk hematologic malignancy were eligible to receive UCB-derived Tregs if they met the following criteria: 12-70 years of age with an available HLA 4-6/6 UCB graft containing ≥ 3.0 × 107 nucleated cells/kg, suitable organ function for a nonmyeloablative regimen, and free of progressive fungal infection. Eligibility criteria for nonmyeloablative conditioning and choice of partially HLA-matched UCB units have been previously described.15 In this study, all patients (with one exception) received 2 UCB units as the HSC graft. Because of the potential increased risk of sustained dual chimerism after DUCBT, graft units were ABO-compatible. The methodology of HLA typing has been detailed previously.14
Treatment and supportive care
All patients received a conditioning regimen consisting of cyclophosphamide 50 mg/kg on day −6, fludarabine 40 mg/m2 daily on days −6 to −2, and a single fraction of TBI 200 cGy without shielding on day −1. All patients received UCB followed by granulocyte-colony stimulating factor (Neupogen; Amgen) 5 μg/kg daily starting on day +1 until an absolute neutrophil count > 2500/μL was observed for 2 consecutive days. Patients received mycophenolate mofetil (MMF) at 1.5 g intravenously or orally twice daily from day −3 to +30 in combination with cyclosporine (CsA) twice daily with target trough levels of 200-400 ng/mL or, in a subsequent cohort, sirolimus with a loading dose of 12 mg followed by 4 mg daily and a target trough level between 3-12 μg/mL from day −3 to day +100. Tapering was accomplished during the course of 8-12 weeks unless GVHD was diagnosed. Units were thawed by the use of the method described by Rubinstein et al.18 The second UCB unit was infused within 30 minutes of the first UCB unit infusion.
Supportive care followed guidelines as previously reported.15 In brief, patients were hospitalized in single rooms ventilated with high-efficiency particulate air filtration systems and received prophylactic acyclovir, fluconazole, or voriconazole and an extended-spectrum fluoroquinolone as clinically indicated. Documented cytomegalovirus reactivation or infection demonstrated by antigenemia or DNA polymerase chain reaction (PCR) testing after transplantation was treated with therapeutic doses of ganciclovir and intravenous immunoglobulin as indicated. Broad-spectrum antibiotics were administered for fever during neutropenia, and antifungal coverage was added for persistent fever unresponsive to antibiotic therapy. Trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis jiroveci was started after engraftment and continued for 12 months after transplantation. Before Treg infusion, all patients received intravenous hydration and were premedicated with acetaminophen and diphenhydramine. No steroids were allowed starting 24 hours before the Treg infusion.
Study design
This was a single-center, open-label, phase 1 “fast-track” dose-escalation trial designed to assess the safety profile and maximal tolerated dose (MTD) of ex vivo expanded/activated UCB-derived Tregs.19 Dose levels were 1, 3, 10, or 30 × 105 Tregs/kg actual body weight on day +1 with an additional cohort who received a second dose of 30 × 105 Tregs/kg on day +15 after UCBT (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Dose escalation occurred after one patient unless a dose-limiting toxicity (DLT) was observed or for the lack of sufficient expansion of the Treg product to meet the planned dose. If a patient experienced a DLT, additional patients would have been enrolled at that dose level before further dose escalation in a conventional 3 + 3 design. If 2 of 6 patients experienced a DLT, the MTD would have been exceeded. There was no intrapatient dose escalation. Because in our previous experience with UCBT at this institution we incorporated CsA immunoprophylaxis, the MTD was first determined in patients concomitantly receiving CsA. Because CsA has been shown to potentially interfere with optimal Treg function and survival,20-23 the last cohort received Tregs at the maximal dose but in the presence of MMF and sirolimus rather than CsA (supplemental Figure 1)
The protocol was approved by the Institutional Review Board of the University of Minnesota and registered at http://www.clinicaltrials.gov as NCT00602693. All patients provided written informed consent in accordance with the principles of the Declaration of Helsinki before enrollment.
Treg manufacture
Tregs were isolated from a partially HLA-matched third UCB unit (generously provided at a reduced cost by The New York Blood Center) that was 4-6/6 HLA matched to the patient. Donor suitability was determined by current good tissue practices. Because the Treg unit was not expected to persist long term, no interunit matching was required between the Treg donor unit and the 2 HSC graft units. Institutional standard operating procedures were followed to avoid cross-contamination. The UCB unit was thawed and processed according to standard procedures (ie, modified New York Blood Center/Rubinstein et al18 ) in a 37°C sterile saline bath with the use of 10% dextran 40/5% human serum albumin as a wash solution. A MgCl2/rHuDNAse/sodium citrate cocktail was used to prevent clumping before a during the immunomagnetic selection. Enrichment of CD25+ cells was accomplished by positive selection with directly conjugated anti-CD25 magnetic microbeads (Miltenyi Biotec) and the CliniMACS device.24 After the column selection CD25+ cells were suspended at a concentration of approximately 1 × 106 cells/mL in X-VIVO 15 (Cambrex BioScience) supplemented with 10% human AB serum, heat-inactivated (Valley Biomedical Products and Services, Inc), l-glutamine (2mM), n-acetylcysteine (2 mg/mL), and 2.5 mL of gentamicin (10 mg/mL) in a tissue culture flask (37°C/5% CO2). The resultant population contained a median of 86 ± 10.1% CD4+/CD25+ cells by flow cytometry. Isolated cells were subsequently cultured with anti-CD3/anti-CD28 monoclonal antibody-coated Dynabeads (provided under Food and Drug Administration Investigational New Drug #6675 by Dr Carl June, University of Pennsylvania) at a 3:1 bead to cell ratio for 18 ± 1 days.
On day 3, cultures were supplemented with 300 IU/mL IL-2 (Proleukin; Chiron Corporation). Cells were maintained at a density of 5.0 × 105 viable nucleated cells/mL by splitting every 48-72 hours for 14 days and then maintained at a density of 1.0 × 106 nucleated cells/mL until harvesting. All products passed lot release criteria that included: 7-aminoactinomycin D viability ≥ 70%, CD4+CD25+ purity ≥ 60%, < 10% CD4−/CD8+ cells, anti-CD3/anti-CD28 mAB bead count < 100 per 3 × 106 cells, gram stain with “no organisms,” and endotoxin < 5 EU/kg. Remaining cells were frozen with the use of a standard cryopreservative cocktail with Plasma-Lyte A (Baxter Healthcare Corp), dimethylsulfoxide (final concentration 10%), and human serum albumin and stored in Cryocyte bags (Baxter Healthcare Corp), for the planned second infusion on day +15. For the 14 patients receiving a second infusion at day +15, cells were thawed, diluted with 5% albumin/10% Dextran 40, and underwent limited additional lot release testing that included acceptable prefreeze lot release testing results and postthaw acridine orange/propidium iodine viability > 50%; all frozen products met lot release criteria. In 6 products the expansion failed to achieve the target dose level, which required the patients to be treated at a lower dose level.
Chimerism
Chimerism was determined serially on peripheral blood at days +2, +4, +7, +14, +21, +60, +100, and +365 after UCBT as described.14 Quantitative PCR of informative polymorphic variable number tandem repeat or short tandem repeat regions in the recipient and donor was performed as separated neutrophil and mononuclear fractions if the total white blood cells was greater than 1000/μL. DNA was amplified with fluorescent PCR primers for markers found to distinguish the 3 UCB donors and recipient alleles. Fluorescent PCR products were separated by the use of an Applied Biosystems 373 Sequencer or an Applied Biosystems 3100 Genetic Analyzer (Applied Biosystems), and GeneScan software (Applied Biosystems) was used to correlate allele peak areas to the percentage of donor or recipient DNA (accuracy +5%).
Flow cytometry
Flow cytometric studies were performed by the use of standard procedures. In brief, peripheral blood mononuclear cells (PBMNCs) were incubated with antibodies to surface antigens (CD4, CD25, CD127; purchased from BD Biosciences) for 30 minutes at 4°C then washed. Staining for Foxp3 was performed with the use of antihuman FoxP3 Flow Kit (BioLegend) by following the manufacturer's instructions. The detection of donor-derived Tregs was determined by the expression of Treg donor-specific HLA markers before conditioning (T0), at 4 hours after Treg product infusion (T4), and days +2, +4, +7, and +14 days from UCBT. Flow studies were performed on a minimum of 5000 CD4+ event acquired in a FACSCalibur flow cytometer (BD Biosciences), and the analysis was performed with FlowJo software (Treestar Inc).
Suppression assays
A total of 5 × 104 CD4+CD25− T responder cells (Tresp) and 1 × 104 monocyte-derived dendritic cells (MoDCs) stimulator antigen-presenting cells per well in 96-well U-bottom plates. Tresp and MoDCs were purified from adult peripheral blood as previously reported.1 Expanded Treg were added to Tresp/MoDCs in a graded fashion from 1:1 to 1:64 (ie, 5 × 104 to 0.08 × 104 Treg) in a total volume of 200 μL of Treg culture media. Wells were pulsed on days 4-6 with 3H-thymidine (1 Curie; 3.7 104 becquerels) for 16-18 hours. Suppression was assessed with serial dilutions of Tregs from 1:1 to 1:64. Each time point had 6 replicates. Results were expressed in counts per minute. Data were collected with a direct counter (no liquid scintillation). A carboxyfluorescein diacetate succinimidyl ester–based suppression assay also was used in which human PBMNCs were labeled with carboxyfluorescein diacetate succinimidyl ester according to the manufacturer's instructions (Invitrogen) and plated at 105 per well in 96-well U-bottom plates with graded titrations of expanded Tregs (1:2 to 1:32 Treg:PBMNCs) in 200 μL of Treg culture media. Cells were stimulated with anti-CD3 conjugated Dynal Magnetic Beads (Invitrogen) at a bead/PBMNC ratio of 1:1, and on day 4 cells were harvested and stained with antibodies to CD8. Acquired data were analyzed by the use of the proliferation platform in FlowJo software (Treestar Inc).
End points and definitions
The primary end point was the safety and tolerability of the infusion of UCB-derived Tregs. Patients were monitored for 48 hours after the infusion of the Treg product for infusional toxicity and adverse events, which were classified according to National Cancer Institute's Common Terminology Criteria for Adverse Events V3.0 (http://ctep.cancer.gov). DLT was defined as any grade 4 to 5 or any related grade 3 toxicity that occurred within 48 hours of infusion with the exception of hematologic toxicities, fever alone, and common toxicities known to the related to the transplant procedure (eg, neutropenia). Any grade ≥ 2 neurotoxicity that occurred within 48 hours of infusion and sustained > 1 week required reporting. Secondary end points included the description of the kinetics of circulating Tregs as measured at 4 hours after Treg product infusion and on days +2, +4, +7, and +14 days after UCBT (and repeated in patients who received a second infusion); proportion of patients with sustained donor engraftment; proportion of patients with early and sustained dual chimerism; speed and cumulative incidence of sustained donor-derived neutrophil engraftment by day +42 and platelet recovery by 6 months' UCBT; grades II-IV and III-IV aGVHD at day +100; chronic GVHD at 1 year; relapse at 1 year, nonrelapse mortality (NRM) at day +100 and at 1 year; and opportunist infections at day +100, as well as probability of disease-free survival (DFS) at 100 days and 1 year after UCBT. Sustained donor engraftment was defined as the detection of ≥ 5% chimerism in the presence of an ANC ≥ 500/μL. The proportion of patients with dual UCB chimerism (ie, detection of progeny from both HSC units) was determined by the detection of ≥ 5% of the 2 donor units by chimerism assay at day +21. Neutrophil recovery was defined as the first of 3 consecutive days with an ANC ≥ 500/μL. Platelet recovery was defined as the first of 7 days of an unsupported platelet count ≥ 50 000/μL Diagnosis of aGVHD was made within 100 days and chronic GVHD thereafter and was determined by standard clinical criteria with histopathologic confirmation when possible.25,26 Relapse was defined as pathologic or imaging evidence of disease recurrence or progression. Opportunistic infections were defined as any culture, PCR, or radiographic image consistent with viral and fungal infections. NRM was defined as death by any cause in the absence of disease relapse. DFS was defined as patients who were alive and without evidence of disease.
Statistical analysis
Estimations of toxicity rates, neutrophil and platelet recovery, GVHD, chimerism, DFS, and the proportion of patients with detectable circulating Tregs were descriptive. DFS was estimated by Kaplan-Meier,27 and neutrophil and platelet recovery, engraftment, GVHD, and NRM were estimated by cumulative incidence.28 The Spearman rank correlation was used to study correlations between non-normally distributed variables.29 To assess potential alterations in risks of graft failure, GVHD, NRM, and survival after Treg infusion, we compared outcomes in 108 adults hematologic malignancies treated in an identical fashion by using the same nonmyeloablative conditioning with CsA and MMF immunoprophylaxis. Data analysis was conducted in August 2010.
Results
Patient, graft, and Treg characteristics
Twenty-three patients, median age of 52 years (range, 24-68 years) and median weight 77 kg (range, 50-133 kg), were treated between September 2007 and October 2009. Diagnoses were acute myeloid leukemia (n = 8), acute lymphoblastic leukemia (n = 3), lymphoma (n = 8), chronic lymphocytic leukemia (n = 3), and prolymphocytic leukemia (n = 1). All but 1 patient received 2 partially HLA matched UCB units with median total nucleated cell dose of 4.1 × 107/kg (range, 2.4-10.0 kg), CD34+ dose of 5.0 × 105/kg (range, 2.3-10.7 kg), and CD3 dose of 1.6 × 107/kg (range, 0.9-3.6 kg). UCB units were 6/6 HLA matched (13%), 5/6 matched (63%), or 4/6 matched (24%). Demographic characteristics of Treg and 108 historical control patients are summarized in the supplemental Table 1.
UCB units identified for Treg manufacture contained a median of 16 × 108 total nucleated cells (range, 0.8-3.4) at the time of thawing. Cell recoveries pre- and post-CD25+ selection, ex vivo expansion culture, and at infusion are summarized in Table 1. The median expansion was 211-fold (range, 13-1796-fold). After CD25+ selection, the median proportion of CD4+CD25+ cells was 65% (range, 11%-87%). After expansion culture, the median proportion of CD4+CD25+, CD4+CD25bright, CD4+CD45RA+, and CD4+CD127−FoxP3+ was 86% (range, 62%-97%), 75% (range, 69%-79%), 92% (range, 69%-97%) and 64% (range, 31%-96%; supplemental Figure 2), respectively.
All Treg products were suppressive in vitro with median suppression at the end of the culture period of 86% (range, 39%-95%) at a 1:4 ratio with an inverse correlation between postexpansion absolute number of CD4+/CD25+ cells and the level of suppression (R = −0.48, P = .03). In contrast, there was no correlation between the absolute number or proportion of CD4+/CD25+ cells before expansion and proportion of CD4+/CD25+ cells after culture, fold expansion, and degree of suppression (data not shown).
The infused Treg dose for individual patients and reasons for not receiving the targeted dose are summarized in Table 2. Seventeen of 23 (74%) received the Treg infusion at the target cell dose level. Five patients received a Treg dose less than the prescribed cell dose because of insufficient culture expansion. Of the 18 patients scheduled to receive 2 doses, 13 received the targeted Treg dose of 30 × 105/kg on days +1 and +15, 1 patient received 2 Treg doses of 21 × 105/kg (less than the planned dose because of insufficient culture expansion), and 1 patient received a lower-than-prescribed dose because of peritransplant morbidities before infusion on day +15. Overall, 18 patients received a total Treg dose > 30 × 105/kg.
MTD: Treg infusional toxicity profile and detection kinetics
Infusion-related toxicity.
No DLT was observed from Treg infusions. There were 2 patients with grade 3 hypertension, one after infusion of a fresh and 2 after infusion of cryopreserved Treg product, with all resolving with standard clinical management. Two patients had grade 2 neurologic changes before the infusion that were attributed to previously prescribed narcotic medication. The infusional toxicity profile is summarized in Table 3.
Detection of UCB-derived Treg after infusion.
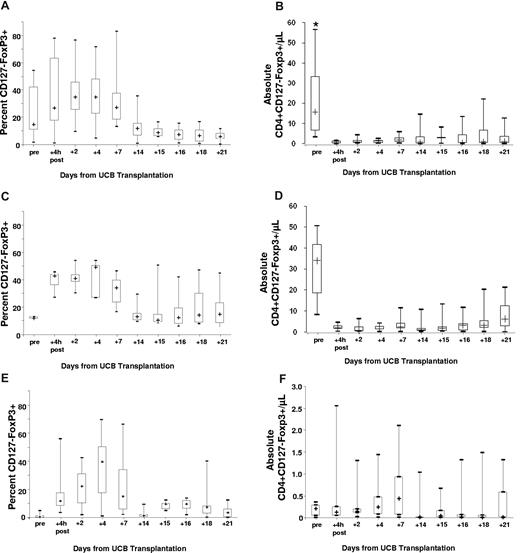
In the 23 patients studied, the median proportion of peripheral CD4+ T cells that was CD127−FoxP3+ was 27% (range, 1%-78%) at 4 hours after infusion (T4), 30% (range, 8%-77%) on day +2, 27% (range, 5%-72%) on day +4, 21% (range, 7%-83%) on day +7, and 10% (range, 1%-36%) on day +14. Notably, among the 18 patients who received ≥ 30 × 105/kg Tregs, the proportion of CD4+ cells that were CD127−FoxP3+ and absolute numbers of peripheral blood CD4+CD127−FoxP3+ cells were not statistically different at all time points in recipients of CsA (n = 12, Figure 1A-B) versus sirolimus (n = 6, Figure 1C-D). Specifically, the proportion of CD4+ cell that were CD127−FoxP3+ cells in recipients of CsA versus sirolimus was 27% (range, 1%-78%) versus 43% (range, 27%-46%) at +4 hours, 35% (range, 10%-77%) versus 41% (range, 30%-54%) on day +2, 35% (range, 5%-71%) versus 49% (range, 27%-54%) on day +4, 27% (range, 13%-83%) versus 34% (range, 17%-47%) on day +7, and 12% (range, 1%-36%) versus 13% (range, 9%-29%) on day +14 after UCB transplantation. As shown in Figure 1A-F, the proportion of CD4+ cells that were CD127−FoxP3+ and the absolute number of CD4+CD127−FoxP3+ cells in the peripheral blood of the cryopreserved product infused on day +15 (CsA n = 8; sirolimus n = 6) was lower compared with freshly infused Tregs on day +1.
Box-plots show the kinetics of CD127-Foxp3+ cells. Proportions and absolute numbers of peripheral blood Treg were determined before and after the infusion of fresh culture-expanded UCB Tregs that were administered 1 day after UCB transplantation (all patients) and again, before and after the infusion of thawed UCB Treg (same donor) 15 days after UCB transplantation (11/17 recipients of CsA/MMF and 6/6 recipients of sirolimus/MMF). In the plot, the cross represents the median value, the box represents the interquartile range, and the vertical line represents the range of the results. Panels A and B show the proportion and absolute number per microliter of peripheral blood CD127-Foxp3+ cells within the CD4+ subset, specifically in recipients of CsA/MMF. Panels C and D show the proportion and absolute number per microliter of peripheral blood CD127-Foxp3+ cells within the CD4+ subset, specifically in recipients of sirolimus/MMF. In a subgroup of 7 patients who had informative HLA discrepancies and received infusions on day +1 and +15 that could be studied by flow cytometry depicting the proportion (E) and absolute numbers per microliter (F) of peripheral blood CD4+CD127−Foxp3+ cells derived from the regulatory T-cell donor unit. (*) In panel B, at the pretransplantation time point the figure excludes one outlier with an absolute Treg count of 152/μL.
Box-plots show the kinetics of CD127-Foxp3+ cells. Proportions and absolute numbers of peripheral blood Treg were determined before and after the infusion of fresh culture-expanded UCB Tregs that were administered 1 day after UCB transplantation (all patients) and again, before and after the infusion of thawed UCB Treg (same donor) 15 days after UCB transplantation (11/17 recipients of CsA/MMF and 6/6 recipients of sirolimus/MMF). In the plot, the cross represents the median value, the box represents the interquartile range, and the vertical line represents the range of the results. Panels A and B show the proportion and absolute number per microliter of peripheral blood CD127-Foxp3+ cells within the CD4+ subset, specifically in recipients of CsA/MMF. Panels C and D show the proportion and absolute number per microliter of peripheral blood CD127-Foxp3+ cells within the CD4+ subset, specifically in recipients of sirolimus/MMF. In a subgroup of 7 patients who had informative HLA discrepancies and received infusions on day +1 and +15 that could be studied by flow cytometry depicting the proportion (E) and absolute numbers per microliter (F) of peripheral blood CD4+CD127−Foxp3+ cells derived from the regulatory T-cell donor unit. (*) In panel B, at the pretransplantation time point the figure excludes one outlier with an absolute Treg count of 152/μL.
As expected, Tregs circulating after transplant were derived from the patient and HSC UCB graft as well as the manufactured Treg product. On the basis of the informative HLA marker on the UCB-Treg unit we were able to track the ex vivo–expanded Tregs after infusion in 7 patients (Figure 1E-F). The proportion of product-derived Tregs in the peripheral blood for all patients peaked on day +2 at 30% (range, 8%-77%) and for those who received Tregs at a dose ≥ 30 × 105/kg at day +4, representing up to 40% (range, 5%-72%) of all Tregs in the circulation. UCB-derived Tregs were detected for up to 14 days with no difference in the kinetics in recipients of CsA/MMF versus sirolimus/MMF (Figure 1A-F)
Impact on transplant outcomes
Opportunistic infections.
Because of the potential of Tregs to increase the risk of opportunistic infection and/or relapse, patients were monitored for either outcome. The cumulative incidence of opportunistic infections (viral and fungal) by day +100 was 39% (95% confidence interval [CI], 19%-59%) for all Treg treated patients and 44% (95% CI, 22%-67%) for those who received ≥ 30 × 105/kg. Notably, the incidence of opportunistic infection was similar in 108 historical control patients treated with an identical conditioning regimen and CsA/MMF posttransplant immunosuppression (53%; 95% CI, 42%-63%). In Treg recipients the incidence of fungal infection was 9% (95% CI, 0%-20%). For those who received a total Treg dose of ≥ 30 × 105/kg (n = 18), the incidence of fungal infection was 11% (95% CI, 0%-25%). The incidence of fungal infections in 108 historical controls was 23% (95% CI, 15%-31%; P = nonsignificant [NS]). One patient had Candida glabrata and Aspergillus niger found during a bronchoscopy, one patient had C glabrata in sputum culture, and another patient had Saccharomyces cerevisiae in a stool culture. In Treg recipients the incidence of viral infection was 35% (95% CI, 16%-54%). For those who received a total Treg dose of ≥ 30 × 105/kg (n = 18), the incidence of viral infection was 39% (95% CI, 17%-61%). The incidence of viral infections in 108 historical control patients was 45% (95% CI, 35%-55%; P = NS). Viremia with human herpes virus 6 was detected in 8 patients and cytomegalovirus in 2 patients. Four patients had positive viral cultures from nasopharyngeal swabs with parainfluenza 3 (n = 2), respiratory syncytial virus (n = 1), and influenza A (n = 1). Despite potential immunosuppressive properties of ex vivo–expanded UCB-derived Tregs, the risk of opportunistic infection was not adversely affected.
Relapse.
Eight of 23 Treg-treated patients relapsed (Table 3), with a median follow-up of living patients of 368 days (range, 226-388 days), and the cumulative incidence of relapse of 32% (95% CI, 13%-52%). For patients who received a total Treg dose of ≥ 30 × 105/kg, the incidence of relapse was 23% (95% CI, 4%-42%; Table 4). Relapses occurred in 2 of 8 with lymphoma, 4 of 8 patients with acute myeloid leukemia, and 2 of 3 with acute lymphoblastic leukemia. None of the 3 chronic lymphocytic leukemia patients or the one patient with prolymphocytic leukemia relapsed. Compared with 108 historical controls, 50% (95% CI, 39%-60%) of whom relapsed, there was no increased relapse resulting from the addition of Tregs (P = NS), which is consistent with results from preclinical models.30
Survival and engraftment.
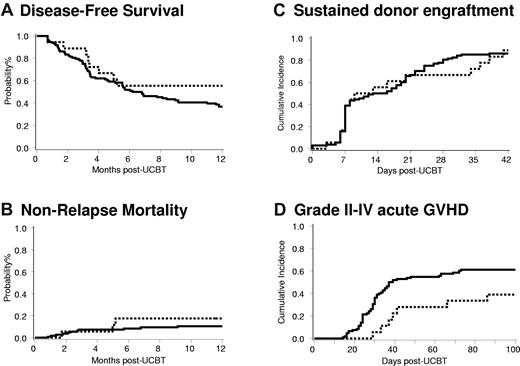
DFS at 1 year was 41% (95% CI, 19%-62%) for all Treg-treated patients and 58% (95% CI, 31-78) for those who received a total Treg dose of ≥ 30 × 105/kg (Figure 2A). NRM at 100 days was 9% (95% CI, 0%-20%) for all patients, whereas it was 6% (95% CI, 0%-16%) for those patients who received Tregs at a total dose ≥ 30 × 105/kg (Figure 2B, Table 4). The primary causes of NRM in Treg-treated patients were bacterial infection (n = 3), aGVHD (n = 1), and multiple organ failure (n = 1). These results are similar to 108 historical controls for whom a DFS at 1 year was 37% (95% CI, 28-46; P = NS) and NRM was 7% (95% CI, 2%-12%; P = NS).
Clinical outcomes of patients after nonmyeloablative umbilical cord blood transplantation who received Treg ≥ 30 × 105/kg (dotted line; n = 18) and historical controls (solid line; n = 108). (A) DFS (P = .25), (B) NRM (P = .51), (C) sustained donor engraftment (P = .89), and (D) grade II-IV aGVHD (P = .04).
Clinical outcomes of patients after nonmyeloablative umbilical cord blood transplantation who received Treg ≥ 30 × 105/kg (dotted line; n = 18) and historical controls (solid line; n = 108). (A) DFS (P = .25), (B) NRM (P = .51), (C) sustained donor engraftment (P = .89), and (D) grade II-IV aGVHD (P = .04).
Sustained donor-derived neutrophil engraftment was observed in 87% (95% CI, 70%-97%) in recipients of Treg and 89% (95% CI, 70%-98%) for those patients who received a Treg dose ≥ 30 × 105/kg (Figure 2C). Of the 2 patients who had graft failure, both had human herpes virus 6 viremia. One patient recovered counts at day +51 with donor-derived hematopoiesis without further intervention; the second patient received a second UCBT at day +60. The incidence of platelet recovery in Treg-treated patients by day +100 was 74% (95% CI, 51%-97%) at a median of 46 days (range, 27-87 days) and for those patients who received a Treg dose ≥ 30 × 105/kg was 78% (95% CI, 53%-100%) at a median of 43 days (range, 29-83 days). Neither sustained donor engraftment nor platelet recovery was adversely affected by Tregs compared with 108 historical controls, in whom recoveries were 86% (95% CI, 79%-92%; P = NS) and 67% (95% CI, 56%-78%; P = NS), respectively.
The median marrow chimerism in Treg-treated patients at day +21 was 91% (range, 37%-100%) with 12 of 21 (57%) engrafted patients having dual chimerism. The prevalence of dual chimerism after Treg treatment tended to be greater than that observed in 108 historical controls, which was 36% (P = .06). By day +100, 2 patients (11%), both receiving a Treg dose of 30 × 105/kg on days +1 and +15 with CsA/MMF immunosuppression, had persistent dual chimerism, which is similar to that observed in 108 historical controls (11%, P = .68). Although immunologic properties of infused UCB Tregs may be promoting early dual chimerism, the pattern of long-term single donor chimerism is unchanged.
GVHD
The incidence of grades II-IV aGVHD for all Treg-treated patients was 43% (95% CI, 23%-64%) at a median time of 38 days (range, 24-86 days) and was similar in those who received a total Treg dose ≥ 30 × 105/kg (39%; 95% CI, 16%-61%) at a median of 39 days (range, 29-86 days; Figure 2D;Table 4). This finding was lower than that observed in 108 historical controls (61%; 95% CI, 51%-72%) at a median of 30 days (range, 14-73 days) compared with all Treg recipients (n = .05) and with recipients who received a total Treg dose ≥ 30 × 105/kg (n = .04). The incidence of grades III-IV acute GVHD for all Treg-treated patients was 17% (95% CI, 2%-23%) at a median time of 51.5 days (range, 24-86 days), and for those who received a Treg dose ≥ 30 × 105/kg, it was 11% (95% CI, 0%-25%) at a median time of 76 days (range, 66-86 days). This finding was similar to that observed in the 108 historical controls (23%; 95% CI, 15%-31% at a median of 29 days; range, 14-72 days; P = NS). Two of 14 (14%) Treg patients at risk developed chronic GVHD. Thus far, chronic GVHD has not been observed among patients who received a total Treg dose ≥ 30 × 105/kg. These results compare favorably with 108 historical controls in whom the incidence of chronic GVHD was 26% (95% CI, 17%-35%). Taken together, these data support further investigation to determine whether ex vivo–expanded and –activated UCB Tregs can suppress GVHD.
Discussion
This is the first report on the use of ex vivo–expanded, –activated, UCB-derived Tregs in humans. By following studies in murine models that demonstrated the potential of natural Tregs for preventing aGVHD and autoimmune disease, we developed a method for isolating and expanding natural Tregs under cyclic guanosine monophosphate conditions.24 UCB was a particularly attractive initial source because of the relatively high proportion of circulating natural Tregs and paucity of T memory cells in the term fetus compared with adult peripheral blood.31,32 This difference had significant implications in the process of selection and expansion methodology. Although multiple selection steps followed by culture in the presence of sirolimus were required for adult peripheral blood Treg,33 expansion of UCB-derived Tregs obtained after only one CD25 selection step did not require the administration of sirolimus to inhibit T effector cells outgrowth. In this phase 1 clinical trial, we demonstrated the safety and feasibility (in the majority of patients) of the adoptive transfer of up to 30 × 105/kg UCB-derived Tregs (administered once or twice during a 2-week period).
Of importance, we observed a variation on the expansion of individual UCB units that may be explained by variation on the reagents or factors inherent to the donor UCB itself. Although all Treg donor units were obtained from a single cord blood bank (New York Blood Center), variations in Treg numbers may be attributable to race, gestational age, and obstetrical history.32,34 Five of 23 Treg products failed to achieve the targeted cell dose. Despite substantial expansion (median 211-fold), all products had > 60% of FoxP3-positive cells, < 10% CD8 T cells, and retained suppressive function. Correlations with UCB unit characteristics such as pre-expansion nucleated or CD25+ cell numbers were not detected. If greater numbers of Tregs are desired, either more efficient separation of Tregs or greater expansion may be achieved with technologic improvements in cell processing.
From a technical perspective, the study demonstrates: (1) the feasibility of manufacturing > 2.4 × 108 Tregs from 80% of the UCB units to provide a cell dose of 30 × 105/kg for an 80-kg recipient, and (2) that the isolation and ex vivo expansion of UCB-derived Tregs reliably results in a CD4+CD127−FoxP3+ product with potent in vitro suppression. From a clinical perspective, the results of the study support the conclusion that UCB-Tregs (1) are safe at the doses used with no obvious effects on the risk of opportunistic infection or relapse, (2) are detectable after infusion with no obvious differences observed in recipients of CsA compared with sirolimus, and (3) transiently increase dual chimerism in the setting of DUCBT. Although we observed a decrease in the risk of aGVHD in Treg recipients, definitive results will require the completion of a planned trial randomizing patients to receive or not receive Tregs.
In addition, in 14 patients we also evaluated the safety of 2 Treg infusions, on day +1 (fresh) and one on day +15 (cryopreserved and thawed) after UCBT. In each patient, there was a marked reduction in the number and duration of circulating Tregs from the manufactured product. Although the mechanism for this difference in unknown, possible reasons for reduced detectability with the second infusion include poorer viability after cryopreservation (although viability studies upon thawing exceeded 50%), presensitization to HLA or other antigens expressed on the Treg product after the first infusion leading to immune rejection, or changes in the microenvironment (eg, lower levels of homeostatic cytokines present in the blood) on day +15 compared with day +1 attributable to emerging lymphohematopoietic engraftment and consumption of cytokines that can drive Treg expansion.
The infusion of the UCB-derived Treg product when patients have therapeutic levels of CsA also may have contributed to the low numbers of circulating cells detected in the CsA subgroup of patients because this drug has been shown to be deleterious to Treg survival.22 In contrast, sirolimus is an immunosuppressive agent that has been shown both in vitro and in vivo to favor the relative expansion of Treg versus effector T cells,35-40 perhaps by suppressing expansion of CD8+ population. In vivo data from a GVHD model showed that, compared with CsA, sirolimus was the immunosuppressive drug least deleterious to Treg survival and expansion.22,41 Therefore, once we determined the safety of the UCB-derived Treg infusion in the setting of CsA/MMF, we modified the immunosuppressive regimen by replacing CsA with sirolimus, which would potentially favor the survival and expansion of the UCB-derived Treg product. An analysis of the 12 CsA/MMF- and 6 sirolimus/MMF-treated patients showed a greater Treg number at some of the early times points postinfusion in the latter, although these differences were not statistically significant in this preliminary study. Nonetheless, this is the first published experience of sirolimus in the context of UCBT. The safety profile of this combination sets the stage for a randomized trial with all patients receiving sirolimus/MMF with or without coadminstration of Tregs on day +1.
As an alternative to ex vivo expansion of Tregs, other investigators are exploring the in vivo activation and expansion of freshly isolated adult peripheral blood Tregs into lymphopenic recipients.42,43 Ultimately, the safety of ex vivo expanded Tregs in this setting has important implications for their use in settings other than hematopoietic cell transplantation. In those settings, relatively high numbers of infused Tregs may be desirable because patients are not lymphopenic and therefore cytokines that may drive Treg expansion may not be present in high quantities in vivo. In the future, when antigen-specific Treg generation has been optimized and/or an in vivo approach to expand Tregs especially in nonlymphopenic patients has been found efficacious, the infused number of expanded Tregs derived from polyclonal activation and expansion conditions may not be as critical as their relative potency and duration of engraftment.
In summary, our results demonstrate the safety of the administration of UCB-derived Tregs at a dose of 30 × 105/kg after nonmyeloablative UCBT. Although the culture methodology used in this clinical trial consistently provided a Treg-cell dose of 30 × 105/kg from a single UCB unit, changes in the manufacturing methodology are required for testing greater Treg doses. However, these preliminary results already suggest that a total Treg dose of 30 × 106/kg may be sufficient for aGVHD prevention and set the stage for a definitive randomized trial that uses UCB Tregs as part of the aGVHD prophylactic regimen.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Marilee Larkin, Elizabeth Kerr, and Jill Aughey from the University of Minnesota Clinical Trials Office CETI for research nurse and regulatory support during the execution of this clinical trial; Diane Kadidlo and Darin Sumstadt from the University of Minnesota Medical Center Cell Therapy Laboratory, Molecular & Cellular Therapeutics for their assistance with cell therapy development and production; Zhaohui Zheng and the Clinical Cell and Vaccine Production Facility of the University of Pennsylvania for production and testing of anti-CD3/anti-CD28 monoclonal antibody beads; and The New York Blood Center for kindly providing the UCB units for Treg manufacture.
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (to C.G.B, J.S.M, D.H.M, P.B.M, B.R.B, J.E.W.), the Children's Cancer Research Fund (to J.E.W., T.E.D.) and R01 CA105216 (to C.H.J.), National Heart, Lung and Blood Institute N01 HB037164 (to J.S.M., D.H.M., J.E.W.) and HHSN268201000008C (J.S.M., D.H.M., K.L.H., J.C., J.E.W.), Leukemia and Lymphoma Translational Research, grant R6029-07 (to B.R.B.), and National Marrow Donor Program grant 13 396 AM#2 (to B.R.B., J.E.W.).
National Institutes of Health
Authorship
Contribution: C.G.B. was involved in the study conception, design, execution and data analysis, the drafting of the article, and final approval of the version to be published; J.S.M. was involved in the study conception, execution and data analysis, manuscript writing, and final approval of the version to be published; Q.C. was involved in the study data analysis, manuscript writing, and final approval of the version to be published; D.H.M. was involved in the Treg manufacture, study execution, manuscript writing, and final approval of the version to be published; K.L.H. was involved in the Treg manufacture, study execution, manuscript writing, and final approval of the version to be published; J.C. was involved in the evaluation of Treg kinetics, study execution, manuscript writing, and final approval of the version to be published; T.D. was involved in the statistical analyses, study design, manuscript writing, and final approval of the version to be published; B.L.L. was involved in the study execution, manuscript writing, and final approval of the version to be published; C.H.J. was involved in the study execution, manuscript writing, and final approval of the version to be published; P.R. was involved in the evaluation of the study results, manuscript writing, and final approval of the version to be published; P.B.M. was involved in the study conception, manuscript writing, and final approval of the version to be published; B.R.B. was involved in the study conception, design, execution and data analysis, manuscript writing, and final approval of the version to be published; and J.E.W. was involved in the study conception, design, Treg manufacture, study execution, data analysis, manuscript writing, and final approval of the version to be published as well as securing the funding for both Treg manufacture and data collection.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Claudio G. Brunstein, Department of Medicine, Mayo Mail Code 480, 420 Delaware St, SE, Minneapolis, MN, 55455; e-mail: bruns072@umn.edu.