Abstract
The clinical outcome of granulocyte transfusion therapy is often hampered by short ex vivo shelf life, inefficiency of recruitment to sites of inflammation, and poor pathogen-killing capability of transplanted neutrophils. Here, using a recently developed mouse granulocyte transfusion model, we revealed that the efficacy of granulocyte transfusion can be significantly increased by elevating intracellular phosphatidylinositol (3,4,5)-trisphosphate signaling with a specific phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670. Neutrophils treated with SF1670 were much sensitive to chemoattractant stimulation. Neutrophil functions, such as phagocytosis, oxidative burst, polarization, and chemotaxis, were augmented after SF1670 treatment. The recruitment of SF1670-pretreated transfused neutrophils to the inflamed peritoneal cavity and lungs was significantly elevated. In addition, transfusion with SF1670-treated neutrophils led to augmented bacteria-killing capability (decreased bacterial burden) in neutropenic recipient mice in both peritonitis and bacterial pneumonia. Consequently, this alleviated the severity of and decreased the mortality of neutropenia-related pneumonia. Together, these observations demonstrate that the innate immune responses can be enhanced and the severity of neutropenia-related infection can be alleviated by augmenting phosphatidylinositol (3,4,5)-trisphosphate in transfused neutrophils with PTEN inhibitor SF1670, providing a therapeutic strategy for improving the efficacy of granulocyte transfusion.
Introduction
Severe infection frequently occurs when the number of neutrophils in the blood is too low (neutropenia).1,2 The blood of healthy adults contains approximately 1500 to 7000 neutrophils/mm3. Neutropenia is defined as an absolute neutrophil count (ANC) of < 1500/mm3 for non–African Americans, or < 1200/mm3 for African Americans. The risk of infection begins to increase at an ANC <1000/mm3. It is considered as severe neutropenia when the ANC falls below 500/mm3. One common cause of severe neutropenia is chemotherapy, which is extensively used to treat various hematologic malignancies and solid tumors. Neutropenia-associated infection is the most important dose-limiting toxicity of this therapeutic treatment, impacting on the quality of life and clinical outcomes, with the potential to cause death.1-3
Neutropenia-related infections have been treated with broad-spectrum antibiotic therapy and granulocyte colony-stimulating factor (G-CSF) therapy. However, not all patients respond to antibiotic treatment. G-CSF therapy often does not work before the bone marrow is recovered and is associated with side effects such as bone pain, headache, fatigue, and nausea.1-4 Granulocyte transfusion also has been considered a therapeutic modality for life-threatening bacterial and fungal infections in severe neutropenic patients.5-8 The possibility of enhancing host immune defenses by infusion of neutrophil has been explored for > 70 years. Numerous studies demonstrated that transfusion of granulocyte concentrates obtained without growth factor stimulation or G-CSF–mobilized neutrophils is of benefit for the treatment of bacterial or fungal infection in neutropenic patients.9-16 However, although large doses of neutrophils can now be easily obtained from healthy donors who have been stimulated with G-CSF, the clinical outcome of granulocyte transfusion therapy is still hampered by short ex vivo shelf life and rapid in vivo death of neutrophils, inefficiency of recruitment to sites of infection, and poor pathogen-killing capability of the transplanted neutrophils. This limits the clinical use of granulocyte transfusion in the treatment of neutropenia-related infections.
Neutrophils are recruited to the site of infection and activated by responding to a variety of chemokines, leukotrienes, complement peptides, and some chemicals released by bacteria directly, such as peptides bearing the N-formyl group (formyl-peptides).17,18 All these responses are mediated by heterotrimeric guanine nucleotide-binding regulatory protein (G protein)–coupled receptors. One of the key pathways downstream of G protein-coupled receptors is mediated by an inositol phospholipid (phosphoinositide), phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3]. The cellular PtdIns(3,4,5)P3 level is regulated by phosphatidylinositol 3′-kinases (phosphatidylinositol 3-kinase or phosphatidylinositol 3-kinase);19-23 the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a phosphatidylinositol 3′-phosphatase that converts PtdIns(3,4,5)P3 to phosphatidylinositol 4,5-bisphosphate;24,25 and Src homology 2-containing inositol 5′ phosphatase [a phosphatidylinositol 5′-phosphatase that converts PtdIns(3,4,5)P3 to phosphoinositide 3-kinase].25,26 A variety of neutrophil functions can be elevated by augmenting cellular PtdIns(3,4,5)P3 signal. For example, depleting PTEN enhances the sensitivity of neutrophil to chemoattractant stimulation, augments neutrophil recruitment to sites of infection, and prevents neutrophil death.27,28 In a neutropenia-related pneumonia model, PTEN-null neutrophils possess an enhanced bacteria-killing capability, and their recruitment to the inflamed lungs is augmented. Moreover, the enhancement of neutrophil function by elevating PtdIns(3,4,5)P3 signaling can alleviate pneumonia-associated lung damage and decrease related mortality rate.29 These results prompted us to ask whether the efficacy of granulocyte transfusion can be improved by targeting PTEN.
In this study, we used a recently developed specific PTEN inhibitor SF1670 to enhance PtdIns(3,4,5)P3 signaling in transplanted neutrophils. We found that treatment with this chemical significantly augmented neutrophil functions such as polarization, phagocytosis, oxidative burst, and recruitment to the sites of infection. As a result, transfusion with neutrophils pretreated with SF1670 ultimately enhanced the inflammatory response and the bacteria-killing capability of neutropenic recipient mice.
Methods
Mice and PTEN inhibitor
Male C57BL/6 mice (8-10 weeks; Charles River Laboratories) were used in all experiments. All procedures involving mice were approved and monitored by the Children's Hospital Animal Care and Use Committee. PTEN inhibitor SF1670 was synthesized based on a published protocol.30
Mouse peritonitis model
Mice were intraperitoneally injected with either 1 mL of 3% thioglycollate ([TG]; Sigma-Aldrich) in distilled water or 500 μL of 1 × 105Escherichia coli (to neutropenic mice) in 0.9% saline. Four to 6 hours after injection, the mice were euthanized by CO2 inhalation. Peritoneal cells were recovered by peritoneal lavage with 5 mL of ice-cold PBS containing 5mM EDTA for 3 times. The total exudate cell counts were determined using a hemacytometer, and the differential cell counts were conducted by microscopic analysis of Wright-Giemsa–stained cytospin. Total number of neutrophils was then determined accordingly. For in vivo bactericidal assay, peritoneal lavage fluid was serially diluted in ice-cold sterile water, and the resulting dilutions (100 μL) were plated on Luria-Bertani agar and incubated at 37°C overnight. Data for viable bacteria were calculated based on the dilution factor and are expressed as CFU per mouse.
Cyclophosphamide-induced mouse neutropenia model
Cyclophosphamide ([Cy]; MP Biomedicals) was injected intraperitoneally at a total dose of 250 mg/kg (150 mg/kg twice on day 1 and 100 mg/kg on day 4, respectively). Blood samples (∼ 30 μL) were taken from the retro-orbital sinuses of anesthetized mice using heparinized capillary tubes (Modulohm A/S) on days 1, 4, 5, 6, and 7. Total and differential white blood cell counts (neutrophils, lymphocytes, and monocytes) were performed using a Hemavet 850 hematology system (Drew-Scientific Inc.). For neutropenia-related inflammation models, mice were challenged with bacteria on day 5 and euthanized at the end of the experiments.
Bacteria-induced acute pneumonia and granulocyte transfusion
Neutropenic mice were anesthetized with ketamine hydrochloride (100 mg/kg IP) and xylazine (10 mg/kg IP). Mouse trachea was surgically exposed, and a total volume of 50 μL of 1 × 105 CFU of E coli (strain 19138; ATCC), 1 × 106 CFU of Streptococcus pneumonia (strain 10342; ATCC), or 1 × 107 CFU of Staphylococcus aureus (strain 10390; ATCC) per mouse was instilled intratracheally to the left bronchus. Four hours after bacteria challenge, 5 million neutrophils pretreated with SF1670 or DMSO(vehicle) were adoptively transferred to the infected mice via tail-vein injection. Mice left without neutrophil adoptive transfer were used as controls. At the end of experiments (24 hours after bacterial infection), mice were euthanized by CO2 inhalation.
Recruitment of adoptive-transferred neutrophils to inflammatory sites
Bone marrow–derived neutrophils were labeled with the dye 5-chloromethylfluorescein diacetate ([CMFDA]; final concentration, 5μM) or the intracellular fluorescent dye 5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine ([CMTMR]; final concentration, 15μM) at 37°C for 10 minutes and then washed twice with PBS. Labeled cells were mixed (1:1) as indicated and then injected intravenously (via tail vein) into neutropenic mice that had been challenged with 3% TG for 2.5 hours. Peritoneal lavage was harvested 1.5 hours after the granulocyte transfusion. The amount of adoptively transferred neutrophils recruited to the peritoneal cavity was analyzed using an FACSCanto II flow cytometer and FACSDiva software (BD Biosciences). Relative recruitment of SF1670-pretreated and untreated neutrophils was calculated as the ratio of indicated populations in the peritoneal cavity.
Assays for neutrophil functions
The related assays, including neutrophil superoxide production, ruffling assay, transwell migration assay, and surface expression of formyl-met-leu-phe (fMLP) receptors are described in previous publications.28,31 Other assays, such as morphometric analyses of histologic lung sections, bronchoalveolar lavage fluid (BALF) collection, bacterial burden, in vitro bactericidal assay, gentamicin protection assay, phagocytosis assay, and phagocytosis assay, are described in detail in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
The Kaplan-Meier and log-rank methods were used to analyze survival rates. Other values were compared using Student t test. Differences were considered significant when the P value was < .05.
Results
Chemoattractant-elicited PtdIns(3,4,5)P3 signaling in neutrophils is augmented by the specific PTEN inhibitor SF1670
We showed previously that depleting PTEN enhances sensitivity of neutrophils to chemoattractant stimulation, augments neutrophil recruitment to sites of infection, and prevents neutrophil death.27,28 Here, we examined whether the efficacy of granulocyte transfusion can be improved by elevating PtdIns(3,4,5)P3 signaling in transfused neutrophils using a specific PTEN inhibitor. PTEN is a member of a large family of cysteine-based phosphatases that also contains protein tyrosine phosphatases (PTPases). Some well-established PTPase inhibitors, such as bisperoxovanadium compounds, were used to inhibit PTEN activity.32,33 However, none of these compounds was regarded specific. In this study, we used a group of recently developed PTEN-specific inhibitors that bind to the active site of PTEN but show little activity against the PTPases.30 Among them, SF1670 seemed to be the most potent and specific compound in elevating PtdIns(3,4,5)P3 signaling in neutrophils.
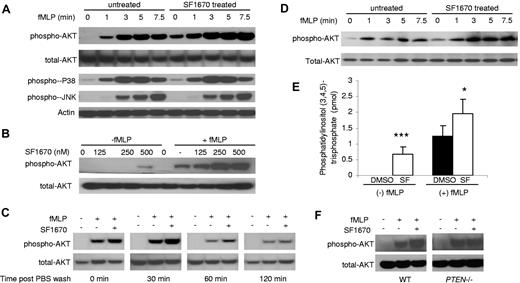
We monitored the effect of PTEN inhibitor on fMLP-elicited PtdIns(3,4,5)P3 signaling by measuring phosphorylation of Akt (Figure 1A). Akt is a serine/threonine protein kinase with oncogenic and antiapoptotic activities. Akt protein possesses a pleckstrin homology (PH) domain that enables it to bind with high affinity to PtdIns(3,4,5)P3 on the cell membrane.34,35 Only the Akt molecules on the plasma membrane can be phosphorylated by 2 phosphatidylinositol-dependent protein kinases and get activated.20,36 Before chemoattractant stimulation, Akt phosphorylation was virtually undetectable in untreated neutrophils. On stimulation, untreated neutrophils showed maximal Akt phosphorylation at 5 minutes, which then declined marginally by 7.5 minutes. Akt phosphorylation was significantly augmented in SF1670-treated human neutrophils at each time point examined, including the basal level, whereas the time course for the increase was not altered (Figure 1A). The effect of SF1670 on PtdIns(3,4,5)P3 signaling seemed to be specific, because fMLP-elicited activations of p38 and Jun were essentially not affected (Figure 1A). In addition, treatment with SF1670 did not significantly affect neutrophil spontaneous death (supplemental Figure 1) and chemoattractant-elicited calcium influx (supplemental Figure 2). The difference in responsiveness could be because of enhanced fMLP receptor expression in SF1670-treated neutrophils. Accordingly, we measured receptor expression on neutrophils. Our result showed that nearly the same amount of fMLP receptor was expressed on SF1670-treated and untreated neutrophils (supplemental Figure 3), suggesting that the increased Akt phosphorylation is a direct consequence of elevated PtdIns(3,4,5)P3.
PTEN inhibitor SF1670 enhances fMLP-induced PtdIns(3,4,5)P3 signaling in both human and mouse neutrophils. (A) Human primary neutrophils were pretreated with 500nM SF1670 at 37°C for 30 minutes and then stimulated with 100nM fMLP for indicated times. The levels of phosphorylated AKT, p38, and c-Jun NH2-terminal kinase (JNK) were assessed by Western blotting with specific antibodies. (B) Human neutrophils were incubated with indicated concentrations of SF1670 at 37°C for 30 minutes and then stimulated with or without 100nM fMLP for 4 minutes. Total and phosphorylated AKT levels were assessed as described in panel A. (C) Human neutrophils were incubated with 500nM SF1670 at 37°C for 30 minutes and then washed twice with PBS. After being incubated in PBS + 2% BSA for indicated times, cells were stimulated with 100nM fMLP for 4 minutes, and then the levels of total and phosphorylated AKT were assessed by Western blotting using specific antibodies. (D) PTEN inhibitor SF1670 enhanced fMLP-induced PtdIns(3,4,5)P3 signaling in mouse neutrophils. Mouse bone marrow derived neutrophils were purified by negative selection using an EasySep system (StemCell Technologies). Cells were pretreated with 500nM SF1670 at 37°C for 30 minutes and then stimulated with 500nM fMLP for indicated times. The levels of phosphorylated AKT were assessed by Western blotting with specific antibody. (E) PtdIns(3,4,5)P3 level increased in SF1670-treated mouse neutrophils. Cellular PtdIns(3,4,5)P3 levels were measured using a mass ELISA kit (Echelon). In brief, PtdIns(3,4,5)P3 was extracted from 5 million purified mouse bone marrow–derived neutrophils following the manufacturer's lipid extraction protocol. The samples were mixed and incubated with a PtdIns(3,4,5)P3 detector protein and then added to a PtdIns(3,4,5)P3-coated microplate for competitive binding. A peroxidase-linked secondary detector and colorimetric detection were used to quantify the PtdIns(3,4,5)P3 detector protein bound to the plate. The colorimetric signal is inversely proportional to the amount of PtdIns(3,4,5)P3 in the sample. (F) Bone marrow–derived neutrophils isolated from wild-type (WT) and myeloid specific PTEN knockout mice28 were preincubated with or without 500nM SF1670 at 37°C for 30 minutes. Cells were then stimulated with or without 1 μM of fMLP for 4 minutes. Total and phosphorylated AKT levels were measured as described in panel A.
PTEN inhibitor SF1670 enhances fMLP-induced PtdIns(3,4,5)P3 signaling in both human and mouse neutrophils. (A) Human primary neutrophils were pretreated with 500nM SF1670 at 37°C for 30 minutes and then stimulated with 100nM fMLP for indicated times. The levels of phosphorylated AKT, p38, and c-Jun NH2-terminal kinase (JNK) were assessed by Western blotting with specific antibodies. (B) Human neutrophils were incubated with indicated concentrations of SF1670 at 37°C for 30 minutes and then stimulated with or without 100nM fMLP for 4 minutes. Total and phosphorylated AKT levels were assessed as described in panel A. (C) Human neutrophils were incubated with 500nM SF1670 at 37°C for 30 minutes and then washed twice with PBS. After being incubated in PBS + 2% BSA for indicated times, cells were stimulated with 100nM fMLP for 4 minutes, and then the levels of total and phosphorylated AKT were assessed by Western blotting using specific antibodies. (D) PTEN inhibitor SF1670 enhanced fMLP-induced PtdIns(3,4,5)P3 signaling in mouse neutrophils. Mouse bone marrow derived neutrophils were purified by negative selection using an EasySep system (StemCell Technologies). Cells were pretreated with 500nM SF1670 at 37°C for 30 minutes and then stimulated with 500nM fMLP for indicated times. The levels of phosphorylated AKT were assessed by Western blotting with specific antibody. (E) PtdIns(3,4,5)P3 level increased in SF1670-treated mouse neutrophils. Cellular PtdIns(3,4,5)P3 levels were measured using a mass ELISA kit (Echelon). In brief, PtdIns(3,4,5)P3 was extracted from 5 million purified mouse bone marrow–derived neutrophils following the manufacturer's lipid extraction protocol. The samples were mixed and incubated with a PtdIns(3,4,5)P3 detector protein and then added to a PtdIns(3,4,5)P3-coated microplate for competitive binding. A peroxidase-linked secondary detector and colorimetric detection were used to quantify the PtdIns(3,4,5)P3 detector protein bound to the plate. The colorimetric signal is inversely proportional to the amount of PtdIns(3,4,5)P3 in the sample. (F) Bone marrow–derived neutrophils isolated from wild-type (WT) and myeloid specific PTEN knockout mice28 were preincubated with or without 500nM SF1670 at 37°C for 30 minutes. Cells were then stimulated with or without 1 μM of fMLP for 4 minutes. Total and phosphorylated AKT levels were measured as described in panel A.
To further assess the potency of SF1670 in elevating PtdIns(3,4,5)P3 signaling, we conducted dose-ranging experiments to estimate the level of SF1670 needed to achieve the maximal effect. SF1670 seemed to be a very potent PTEN inhibitor in neutrophils and could efficiently augment Akt phosphorylation at the concentration of 250nM (Figure 1B). One interesting feature of this PTEN inhibitor is that its inhibitory effect can last as long as 2 hours after the treated neutrophils were washed with drug-free medium (Figure 1C). This prolonged intracellular retention makes SF1670 an ideal candidate for specific inhibition of PTEN and augmentation of PtdIns(3,4,5)P3 signaling in transfused neutrophils, but not in cells of recipients.
SF1670 also elevated Akt phosphorylation in murine cells (Figure 1D). In addition, we measured PtdIns(3,4,5)P3 level directly. Consistent with the enhanced Akt phosphorylation, pretreatment with SF1670 also significantly augmented PtdIns(3,4,5)P3 level in mouse neutrophils (Figure 1E). SF1670-induced Akt hyperactivation was abolished in PTEN-null neutrophils, further demonstrating that this effect was mediated by specific inhibition of PTEN activity (Figure 1F).
Pretreatment with SF1670 enhances neutrophil functions
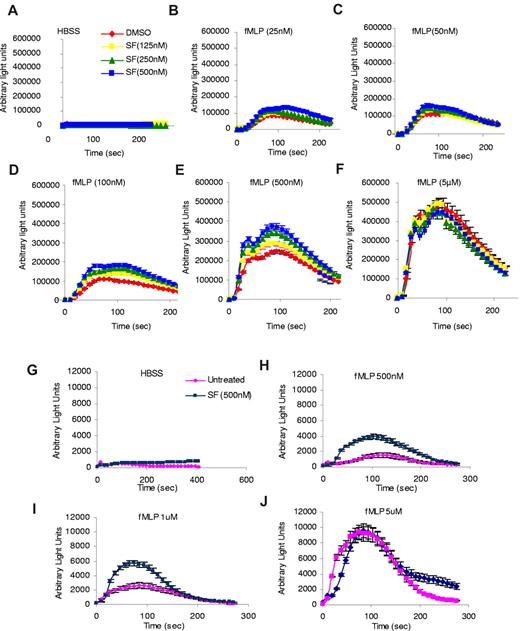
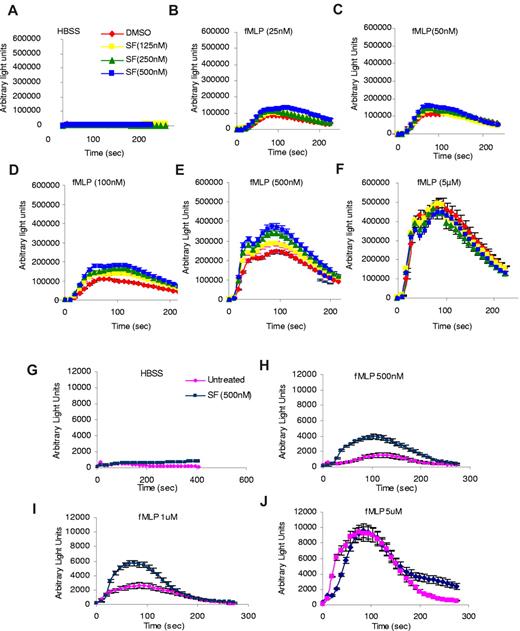
Because SF1670 is a potent and specific PTEN inhibitor with prolonged intracellular retention in neutrophils, we next examined whether pretreatment with this drug can alter neutrophil functions. In the following experiments, neutrophils were pretreated with SF1670 at 37°C for 30 minutes and washed twice with HBSS before each assay. One important downstream effect of chemoattractant-induced PtdIns(3,4,5)P3 production in neutrophils is reduced NADP oxidase-mediated superoxide production. Thus, we first investigated whether pretreatment with SF1670 affects fMLP-elicited superoxide production. Drug-treated and untreated human neutrophils were stimulated with fMLP, and superoxide products were measured using isoluminol chemiluminescence (Figure 2). Significant increase of reactive oxygen species (ROS) production in SF1670-pretreated neutrophils was observed at each fMLP concentration examined, whereas the time course for the increase and subsequent decrease was not altered. The peak production of superoxide occurred at ∼ 100 seconds. It took ∼ 200 seconds to return to the prestimulus level. At 500nM fMLP stimulation, SF1670 (500nM)–pretreated neutrophils showed nearly 70% higher (maximal) superoxide production than untreated neutrophils (Figure 2E). Similar results were obtained in neutrophils stimulated with lower concentrations of fMLP (Figure 2B-C). Interestingly, at high concentrations of fMLP (5μM), both populations showed a similar peak response, indicating that drug-treated and untreated neutrophils were equally capable of responding to fMLP (Figure 2F). The effect of SF1670 on chemoattractant-elicited ROS production also was observed in mouse neutrophils. Similarly, the effect was more prominent when lower levels of fMLP were used (Figure 2G-J).
Treatment with PTEN inhibitor SF1670 increases chemoattractant-elicited superoxide production in both human and mouse neutrophils. (A-F) Human neutrophils were treated with SF1670 at 37°C for 30 minutes and then washed twice with HBSS. Production of ROS was monitored after stimulation with indicated concentrations of fMLP in the presence of 50μM isoluminol, 0.8 U of horseradish peroxidase, and 0.2% bovine serum albumin in a luminometer at 37°C. Chemiluminescence (arbitrary light units) was recorded at indicated times. Data are mean ± SD from 1 experiment representative of 3 experiments. (G-J) Bone marrow–derived mouse neutrophils were treated 500nM SF1670 at 37°C for 30 minutes, washed twice with HBSS, and then stimulated with indicated concentrations of fMLP. ROS production was monitored in the presence of 50μM isoluminol, 0.8 U of horseradish peroxidase, and 0.2% BSA in a luminometer as described here. Data are mean ± SD from 1 experiment representative of 3 experiments.
Treatment with PTEN inhibitor SF1670 increases chemoattractant-elicited superoxide production in both human and mouse neutrophils. (A-F) Human neutrophils were treated with SF1670 at 37°C for 30 minutes and then washed twice with HBSS. Production of ROS was monitored after stimulation with indicated concentrations of fMLP in the presence of 50μM isoluminol, 0.8 U of horseradish peroxidase, and 0.2% bovine serum albumin in a luminometer at 37°C. Chemiluminescence (arbitrary light units) was recorded at indicated times. Data are mean ± SD from 1 experiment representative of 3 experiments. (G-J) Bone marrow–derived mouse neutrophils were treated 500nM SF1670 at 37°C for 30 minutes, washed twice with HBSS, and then stimulated with indicated concentrations of fMLP. ROS production was monitored in the presence of 50μM isoluminol, 0.8 U of horseradish peroxidase, and 0.2% BSA in a luminometer as described here. Data are mean ± SD from 1 experiment representative of 3 experiments.
When neutrophils are uniformly stimulated with a chemoattractant such as fMLP, they display membrane ruffles and polarize, forming distinct pseudopods and uropods. This polarization process is PtdIns(3,4,5)P3-dependent and necessary for the subsequent chemotactic migration and neutrophil recruitment to sites of inflammation. Thus, we next investigated whether pretreatment with SF1670 can alter chemoattractants-induced morphologic changes of neutrophils. Neutrophils were predominantly round before stimulation (Figure 3A). When uniformly stimulated with fMLP, cells polarized (ruffled or formed pseudopodia) in random directions. However, a higher fraction of SF1670 (250nM)–pretreated neutrophils polarized in comparison with untreated cells when fMLP concentration was < 100nM (Figure 3A). When we performed a sensitivity analysis using different concentrations of fMLP, we found that this difference was apparent when fMLP concentration was < 100nM. As the concentration of fMLP was increased, SF1670 induced augmentation of cell polarization became progressively smaller (Figure 3B). This suggests that SF1670-pretreated neutrophils were generally more sensitive to chemoattractant than untreated neutrophils. The different responsiveness was not because of a polarization defect in SF1670-pretreated versus untreated neutrophils, because both neutrophils were equally capable of responding to high concentrations of chemoattractant. In line with the elevation of Akt phosphorylation, SF1670 augmented fMLP-induced neutrophil polarization in a dose-dependent manner, with the maximal effect observed at 500nM (Figure 3C).
Treatment with PTEN inhibitor SF1670 augments fMLP-induced polarization and chemotaxis of mouse neutrophils. (A-C) fMLP induced neutrophil polarization. Bone marrow–derived mouse neutrophils were pretreated with 250nM SF1670 at 37°C for 30 minutes, washed twice with HBSS, and then settled down for 5 minutes on LabTek chamber cover-glass coated with 30 μg/mL bovine fibronectin. Cell polarization was induced by uniform stimulation with indicated concentrations of fMLP. (A) Representative images of cell ruffling (5 minutes after fMLP stimulation). (B) Quantification of fMLP-induced cell ruffling. The percentage of neutrophils extending pseudopods or ruffling was calculated from fields captured 4 to 8 minutes after fMLP stimulation. (C) Cells were pretreated with DMSO (control) or indicated concentrations of SF1670 at 37°C for 30 minutes and then stimulated with 25nM fMLP. The percentage of neutrophils extending pseudopods or ruffling was calculated from fields captured 4 to 8 minutes after fMLP stimulation. (D-G) Chemoattractant-induced transwell chemotaxis of neutrophils. Bone marrow–derived mouse neutrophils were pretreated with indicated amount of SF1670 at 37°C for 30 minutes, washed twice with HBSS, and then allowed to migrate in response to the indicated chemoattractants: fMLP (D), C5a (E), leukotriene B4 (LTB4; F), and macrophage-inflammatory protein-2 (G) in transwell systems. Percentage of cells that migrated into the bottom well was recorded. Data shown are mean ± SD of 6 wells, from 1 experiment representative of 3 experiments.
Treatment with PTEN inhibitor SF1670 augments fMLP-induced polarization and chemotaxis of mouse neutrophils. (A-C) fMLP induced neutrophil polarization. Bone marrow–derived mouse neutrophils were pretreated with 250nM SF1670 at 37°C for 30 minutes, washed twice with HBSS, and then settled down for 5 minutes on LabTek chamber cover-glass coated with 30 μg/mL bovine fibronectin. Cell polarization was induced by uniform stimulation with indicated concentrations of fMLP. (A) Representative images of cell ruffling (5 minutes after fMLP stimulation). (B) Quantification of fMLP-induced cell ruffling. The percentage of neutrophils extending pseudopods or ruffling was calculated from fields captured 4 to 8 minutes after fMLP stimulation. (C) Cells were pretreated with DMSO (control) or indicated concentrations of SF1670 at 37°C for 30 minutes and then stimulated with 25nM fMLP. The percentage of neutrophils extending pseudopods or ruffling was calculated from fields captured 4 to 8 minutes after fMLP stimulation. (D-G) Chemoattractant-induced transwell chemotaxis of neutrophils. Bone marrow–derived mouse neutrophils were pretreated with indicated amount of SF1670 at 37°C for 30 minutes, washed twice with HBSS, and then allowed to migrate in response to the indicated chemoattractants: fMLP (D), C5a (E), leukotriene B4 (LTB4; F), and macrophage-inflammatory protein-2 (G) in transwell systems. Percentage of cells that migrated into the bottom well was recorded. Data shown are mean ± SD of 6 wells, from 1 experiment representative of 3 experiments.
The recruitment of circulating effector leukocytes, including neutrophils, monocytes, and effector T cells to the sites of injury or infection is mediated by a process called chemotaxis, in which cells sense the infection or inflammation and then move up a gradient of molecules (chemoattractants such as chemokines, formyl-peptides, and complement peptides C5a and C3a). A pathway mediated by PtdIns(3,4,5)P3 has been established as one of the essential chemotactic signals.37-39 Therefore, we investigated whether pretreatment with SF1670 can affect neutrophil chemotactic migration using a transwell migration system (Figure 3D-G). Mouse neutrophils were plated on Transwell filters and induced to migrate in response to chemoattractant added to wells beneath the filters. The migration of neutrophils to these lower wells requires 2-dimensional chemotaxis on top of the filter (toward the holes), followed by migration through the holes into the bottom well of chemoattractant. The number of cells in the bottom well was then used to calculate percentage of cells migrated. Consistent with published results, neutrophil responded the best when medium levels of chemoattractant were used.40 However at each fMLP concentration tested, more SF1670-pretreated neutrophils migrated into the lower wells in comparison with untreated neutrophils, even when only media (without chemoattractant) were added to the lower well (Figure 3D). As fMLP concentration in the lower well was varied, both populations of neutrophils exhibited typical bell-shaped migration curves that peaked at 100nM fMLP (Figure 3D). Similarly, other chemoattractants such as C5a (Figure 3E), leukotriene B4 (Figure 3F), and macrophage-inflammatory protein-2 (Figure 3G) also induced the enhanced migration in SF1670-pretreated neutrophils compared with untreated neutrophils. These results are consistent with the higher sensitivity and responsiveness of SF1670-pretreated neutrophils.
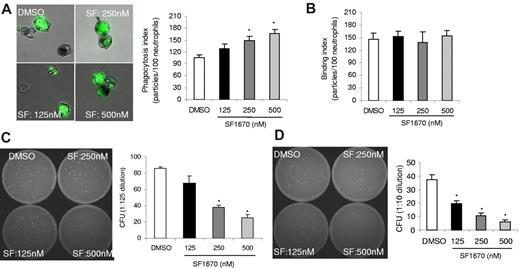
Another important cellular process under the control of PTEN is phagocytosis. Increased phagocytosis was observed in PTEN-null neutrophils and macrophages.29,41 To examine whether pretreatment with SF1670 also can increase the efficiency of this process, we measured neutrophil phagocytosis using an in vitro phagocytosis assay (Figure 4A). Purified mouse neutrophils were pretreated with SF1670, washed, and then incubated with serum-opsonized fluorescein-conjugated zymosan particles. Phagocytic index was calculated as the number of bacteria engulfed by 100 neutrophils. After 1 hour incubation at 37°C, an average of 100 particles was engulfed by 100 untreated neutrophils. Pretreatment with SF1670 increased phagocytic index in a dose-dependent manner. At the concentration of 500nM, nearly 170 zymosan particles were engulfed by 100 neutrophils (Figure 4A). The augmented phagocytosis was probably a result of enhanced engulfment, because there was essentially no difference in the initial zymosan-binding capability between untreated and SF1670-pretreated neutrophils (Figure 4B). Finally, we explored the effect of SF1670 pretreatment on neutrophil bacterial-killing capability. We conducted an in vitro bactericidal assay using SF1670-pretreated and untreated neutrophils. Although SF1670 did not directly affect the growth and survival of bacteria (supplemental Figure 4), SF1670 pretreatment significantly augmented the bacterial-killing capability of neutrophils. At the concentration of 500nM, the bacteria-killing capability of SF1670-pretreated neutrophils was increased by nearly 3-fold, compared with untreated neutrophils (Figure 4C). We also measured neutrophil intracellular bactericidal activity using a gentamicin protection assay. Consistent with the elevated superoxide production, the capability of the SF1670-pretreated neutrophils to kill engulfed bacteria was much increased compared with untreated neutrophils (Figure 4D).
Treatment with PTEN inhibitor SF1670 enhances bacteria-killing capability of neutrophils. (A) In vitro phagocytosis assay. Mouse bone marrow–derived neutrophils were treated with SF1670 at indicated concentrations at 37°C for 30 minutes, washed twice with HBSS, and then incubated with fluorescein isothiocyanate–labeled zymosans (opsonized with mouse serum) at 37°C for 1 hour. Extracellular fluorescence was quenched by trypan blue. Phagocytosis index was expressed as the number of bioparticles engulfed by 100 neutrophils. (B) Binding index was expressed as the number of bioparticles bound to 100 neutrophils. More than 200 neutrophils were counted in each group. Data shown are mean ± SD from 1 experiment representative of 3 experiments. (C) In vitro bactericidal assay. Bone marrow–derived neutrophils were pretreated with indicated amounts of SF1670 as described here, washed twice with HBSS, and then incubated with opsonized live E coli at 37°C. Sterile water was added to lyse neutrophils after 1 hour. Samples were serially diluted and spread on Luria-Bertani agar plates. The numbers of E coli colonies were determined after overnight incubation at 37°C. (D) Gentamicin protection assay. In this experiment, gentamicin was added to kill extracellular bacteria. Data shown are mean ± SD of 3 experiments. *P < .01 vs control.
Treatment with PTEN inhibitor SF1670 enhances bacteria-killing capability of neutrophils. (A) In vitro phagocytosis assay. Mouse bone marrow–derived neutrophils were treated with SF1670 at indicated concentrations at 37°C for 30 minutes, washed twice with HBSS, and then incubated with fluorescein isothiocyanate–labeled zymosans (opsonized with mouse serum) at 37°C for 1 hour. Extracellular fluorescence was quenched by trypan blue. Phagocytosis index was expressed as the number of bioparticles engulfed by 100 neutrophils. (B) Binding index was expressed as the number of bioparticles bound to 100 neutrophils. More than 200 neutrophils were counted in each group. Data shown are mean ± SD from 1 experiment representative of 3 experiments. (C) In vitro bactericidal assay. Bone marrow–derived neutrophils were pretreated with indicated amounts of SF1670 as described here, washed twice with HBSS, and then incubated with opsonized live E coli at 37°C. Sterile water was added to lyse neutrophils after 1 hour. Samples were serially diluted and spread on Luria-Bertani agar plates. The numbers of E coli colonies were determined after overnight incubation at 37°C. (D) Gentamicin protection assay. In this experiment, gentamicin was added to kill extracellular bacteria. Data shown are mean ± SD of 3 experiments. *P < .01 vs control.
Pretreatment with SF1670 augments the recruitment of transfused neutrophils to the site of infection and the bactericidal capability of the host
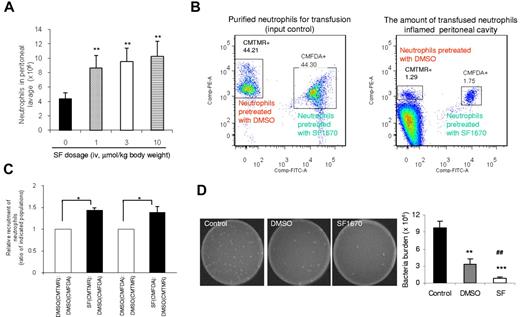
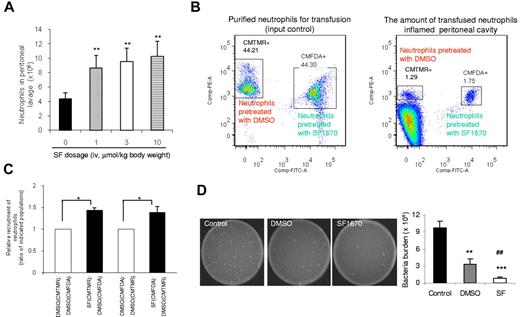
We investigated the role of SF1670 in enhancing neutrophil recruitment to sites of inflammation using a mouse peritonitis model (Figure 5A). Few peritoneal neutrophils were detected in mice injected with PBS. In mice injected with TG, the amount of neutrophils in peritoneal cavity dramatically increased to 4 × 106 at 4 hours after treatment. Intravenous injection of SF1670 significantly elevated the level of neutrophil recruitment. When SF1670 was used at 10 μmol/kg body weight, the TG-induced neutrophil accumulation was increased by nearly 2-fold.
Pretreatment with PTEN inhibitor SF1670 enhances the recruitment of transfused neutrophils to the inflamed peritoneal cavity, as well as the bactericidal capability of the host. (A) Enhanced neutrophil recruitment to inflamed peritoneal cavity in SF1670-treated mice. Mice were intraperitoneally challenged with 1 mL of 3% TG before intravenous injection of the indicated dose of SF1670. Mice were euthanized 4 hours after the challenge. Total neutrophil content in the lavage fluid was calculated by microscopic examination. Data shown are mean ± SD of 3 mice. **P < .01 vs control. (B) Recruitments of adoptively transferred neutrophils to the inflamed peritoneal cavities in neutropenic mice. The neutropenic mice were prepared as described in supplemental Figure 7. Purified mouse neutrophils were pretreated with or without SF1670 (250nM) for 30 minutes at 37°C and then labeled with CMTMR or CMFDA. Labeled cells were mixed (1:1) as indicated and then intravenously injected to neutropenic mice that had been challenged with 3% TG for 2.5 hours. Peritoneal lavage was harvested 1.5 hours after the injection of cell mixture. The amount of adoptively transferred neutrophils recruited to the peritoneal cavity was analyzed using an FACSCanto II flow cytometer and FACSDiva software. (C) Relative recruitment of neutrophils was calculated as the ratio of indicated populations in the peritoneal lavage. Data shown are mean ± SD of 3 mice. *P < .05 vs DMSO-treated neutrophils. (D) In vivo bactericidal assay. Live E coli particles (1.0 × 105 CFU) were injected intraperitoneally to neutropenic mice 4 hours before neutrophil-adoptive transfer. Mouse neutrophils were pretreated with or without SF1670 (250nM) for 30 minutes at 37°C and then washed twice before adoptive transfer. Two hours after transfer, mice were euthanized, and peritoneal lavage was collected to check bacteria loads. **P < .01, ***P < .001 vs control; ##P < .01 vs DMSO.
Pretreatment with PTEN inhibitor SF1670 enhances the recruitment of transfused neutrophils to the inflamed peritoneal cavity, as well as the bactericidal capability of the host. (A) Enhanced neutrophil recruitment to inflamed peritoneal cavity in SF1670-treated mice. Mice were intraperitoneally challenged with 1 mL of 3% TG before intravenous injection of the indicated dose of SF1670. Mice were euthanized 4 hours after the challenge. Total neutrophil content in the lavage fluid was calculated by microscopic examination. Data shown are mean ± SD of 3 mice. **P < .01 vs control. (B) Recruitments of adoptively transferred neutrophils to the inflamed peritoneal cavities in neutropenic mice. The neutropenic mice were prepared as described in supplemental Figure 7. Purified mouse neutrophils were pretreated with or without SF1670 (250nM) for 30 minutes at 37°C and then labeled with CMTMR or CMFDA. Labeled cells were mixed (1:1) as indicated and then intravenously injected to neutropenic mice that had been challenged with 3% TG for 2.5 hours. Peritoneal lavage was harvested 1.5 hours after the injection of cell mixture. The amount of adoptively transferred neutrophils recruited to the peritoneal cavity was analyzed using an FACSCanto II flow cytometer and FACSDiva software. (C) Relative recruitment of neutrophils was calculated as the ratio of indicated populations in the peritoneal lavage. Data shown are mean ± SD of 3 mice. *P < .05 vs DMSO-treated neutrophils. (D) In vivo bactericidal assay. Live E coli particles (1.0 × 105 CFU) were injected intraperitoneally to neutropenic mice 4 hours before neutrophil-adoptive transfer. Mouse neutrophils were pretreated with or without SF1670 (250nM) for 30 minutes at 37°C and then washed twice before adoptive transfer. Two hours after transfer, mice were euthanized, and peritoneal lavage was collected to check bacteria loads. **P < .01, ***P < .001 vs control; ##P < .01 vs DMSO.
To examine whether SF1670 can enhance the recruitment of transfused neutrophils, we first established a mouse model of granulocyte transfusion (supplemental Figure 5). Clinically, granulocyte transfusion is mainly used as a therapeutic approach for life-threatening bacterial and fungal infections in severe neutropenic patients. Accordingly, we conducted experiments using neutropenic recipient mice. One common cause of severe neutropenia is chemotherapy that is extensively used to treat various hematologic malignancies and solid tumors. To mimic the clinical situation, we recently successfully established a mouse model in which neutropenia was induced using a widely used chemotherapeutic drug Cy.29 To induce neutropenia, we injected Cy intraperitoneally at a total dose of 250 mg/kg body weight. On day 5, Cy-treated mice contained approximately 90% fewer circulating neutrophils than untreated or saline-treated group. The profound neutropenia was persisted through days 6 and 7.29 The neutropenic status was confirmed in each experiment conducted in this study (supplemental Figure 6). We next investigated the effect of SF1670 on the recruitment of transfused neutrophils to the inflamed peritoneal cavity. Although neutropenic mice were used as recipient mice, endogenous neutrophils (∼ 10% of the normal level) still existed in these mice and also could migrate to the inflamed peritoneal cavity. Thus, it was necessary to find a way to distinguish the transfused neutrophils from the endogenous neutrophils. To achieve this, we labeled in vitro-purified untreated neutrophils with intracellular fluorescent dye CMTMR (red) and SF1670-treated neutrophils with the dye CMFDA (green), or vice versa. The mixed (1:1) population was intravenously injected into a neutropenic wild-type mouse. By doing this, we were able to compare the neutrophil recruitment of these 2 types of neutrophils in exactly the same environment. Transfused untreated (red) and SF1670-treated (green) neutrophils were identified by their unique fluorescent labels (Figure 5B). Because granulocyte transfusion therapy is mainly applied after infection is identified; to mimic this clinical situation, the peritonitis was induced 2.5 hours before the granulocyte transfusion (supplemental Figure 5A). The relative recruitment rate was calculated by measuring the ratio between treated (green) and untreated (red) neutrophils at the site of inflammation. To further rule out that any observed differences were a result of alterations by the dyes, we also conducted an experiment in which the 2 different dyes were switched (untreated neutrophils were stained with CMFDA and treated neutrophils were stained with CMTMR). We also demonstrated that recruitment of transfused neutrophils to the inflamed peritoneal cavity is independent of the method for neutrophil staining (supplemental Figure 5B-D). No significant difference was observed between CMTMR- and CMFDA labeled neutrophils in their ability to accumulate in the inflamed site (the ratio remained 1 in the peritoneal cavity), whereas we detected a much enhanced peritoneal recruitment of SF1670-pretreated neutrophils compared with untreated neutrophils (Figure 5C). The number of live bacteria left in the inflamed peritoneal cavity (bacterial burden) also was measured. As expected, granulocyte transfusion significantly enhanced the bacteria-killing capability of the neutropenic recipient mice (Figure 5D). Pretreatment of neutrophils with SF1670 further elevate the bacteria-killing capability by > 5-fold (Figure 5D). Collectively, these results demonstrated that SF1670 treatment significantly improved the efficacy of granulocyte transfusion in the bacterial peritonitis model.
Pretreatment with SF1670 increases the efficacy of granulocyte transfusion in a mouse neutropenia-associated bacterial pneumonia model
The ultimate goal of this research is to improve the performance of transfused neutrophils, which is eventually reflected by the recipients' capability of clearing invading pathogens. Bacterial pneumonia is common in neutropenic patients. Thus, we examined whether pretreatment of transfused neutrophils with SF1670 can ultimately enhance the inflammatory response and bacteria-killing capability of the recipient mice in a neutropenia-associated bacterial pneumonia model. We first explored the survival rate of intratracheally instilled live E coli cells in mice transfused with SF1670-pretreated and untreated neutrophils. As expected, because of the lack of neutrophils in the Cy-induced neutropenic mice, a large amount of E coli survived in the lungs. Transfusion with freshly purified neutrophils significantly enhanced the bacteria-killing capability of the neutropenic recipient mice, leading to better clearance of instilled bacteria. Pretreatment of transfused neutrophils with SF1670 further elevated the bacteria-killing capability of the host, as revealed by the reduced bacteria number in the inflamed lungs (Figure 6A). As a result, the resolution of bacteria-induced lung inflammation was accelerated. The inflammation-associated lung damage, assessed by pulmonary edema formation, also was alleviated in mice transfused with SF1670-pretreated neutrophils, compared with mice transfused with untreated neutrophils (Figure 6B-C). Severe pneumonia is often accompanied with vascular leakage. Thus, the elevation of bronchoalveolar lavage fluid protein level has been used as an indicator of vascular leakage and a key parameter of inflammatory lung injury. Consistent with the enhanced bacteria-killing capability and the alleviated lung inflammation, we detected much reduced total protein level in the bronchoalveolar lavage fluid collected from the mice transfused with SF1670-treated neutrophils (Figure 6D).
Pretreatment with SF1670 increases the efficacy of granulocyte transfusion in neutropenia-related pneumonia. (A) Mouse bone marrow–derived neutrophils were pretreated with or without SF1670 ([SF]; 250nM) for 30 minutes at 37°C, washed twice with PBS, and then intravenously injected to neutropenic mice that had been challenged with 105 CFU of E coli for 5 hours. Mice not receiving granulocyte transfusion were used as control. BALF was harvested 24 hours after each challenge. BALF was serially diluted and spread on plates. Colonies were counted next day. **P < .01 vs control; ##P < .01 vs DMSO. (B) Histologic analysis of lungs reveals bacterial colonies and polymerized fibrin in the pulmonary parenchyma (indicated by an arrow). Lung sections were stained with H&E. (C) Pulmonary edema formation was quantified as the percentage of edema in the total parenchymal region using IPLab imaging software as described previously.29 *P < .05, **P < .01 vs control; #P < .05 vs DMSO. (D) BALF total protein level. Protein accumulated in the inflamed lung was measure using a protein assay kit (Bio-Rad Laboratories). *P < .05, **P < .01 vs control; ##P < .01 vs DMSO. (E) Survival rates of mice infected with E coli. Age- and sex-matched neutropenia-related pneumonia mice were adoptively transferred with neutrophils pretreated with or without SF1670. Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. * P < .05 vs DMSO. (F) Pretreatment with SF1670 augments the bactericidal capability of the host in both S pneumoniae- and S aureus-induced neutropenia-related pneumonia. The experiments were conducted essentially as described in panel A. The pneumonia was induced by 106 CFU of S pneumonia or 107 CFU of S aureus. (G) Survival rates of mice infected with S aureus. The experiment was conducted essentially as described in panel E. *P < .05 vs DMSO-pretreated neutrophils.
Pretreatment with SF1670 increases the efficacy of granulocyte transfusion in neutropenia-related pneumonia. (A) Mouse bone marrow–derived neutrophils were pretreated with or without SF1670 ([SF]; 250nM) for 30 minutes at 37°C, washed twice with PBS, and then intravenously injected to neutropenic mice that had been challenged with 105 CFU of E coli for 5 hours. Mice not receiving granulocyte transfusion were used as control. BALF was harvested 24 hours after each challenge. BALF was serially diluted and spread on plates. Colonies were counted next day. **P < .01 vs control; ##P < .01 vs DMSO. (B) Histologic analysis of lungs reveals bacterial colonies and polymerized fibrin in the pulmonary parenchyma (indicated by an arrow). Lung sections were stained with H&E. (C) Pulmonary edema formation was quantified as the percentage of edema in the total parenchymal region using IPLab imaging software as described previously.29 *P < .05, **P < .01 vs control; #P < .05 vs DMSO. (D) BALF total protein level. Protein accumulated in the inflamed lung was measure using a protein assay kit (Bio-Rad Laboratories). *P < .05, **P < .01 vs control; ##P < .01 vs DMSO. (E) Survival rates of mice infected with E coli. Age- and sex-matched neutropenia-related pneumonia mice were adoptively transferred with neutrophils pretreated with or without SF1670. Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. * P < .05 vs DMSO. (F) Pretreatment with SF1670 augments the bactericidal capability of the host in both S pneumoniae- and S aureus-induced neutropenia-related pneumonia. The experiments were conducted essentially as described in panel A. The pneumonia was induced by 106 CFU of S pneumonia or 107 CFU of S aureus. (G) Survival rates of mice infected with S aureus. The experiment was conducted essentially as described in panel E. *P < .05 vs DMSO-pretreated neutrophils.
Lastly, severe neutropenia-related pneumonia can lead to death. Thus, we investigated whether augmentation of the PtdIns(3,4,5)P3 signal by SF1670 in transfused neutrophils can reduce such lethality. Consistent with the increased bacteria-clearance capability, transfusion with SF1670-pretreated neutrophils resulted in a higher survival rate of bacteria-challenged neutropenic mice, compared with mice transfused with untreated neutrophils. Approximately 40% of mice transfused with SF1670-pretreated neutrophils survived. In contrast, only 10% of mice transfused with untreated neutrophils survived (Figure 6E).
Lung infection also can be induced by other pathogens. Thus, we induced neutropenia-related pneumonia with S aureus and S pneumoniae. Similar results were observed in these experiments. The bacteria-killing capability of mice transfused with SF1670-pretreated neutrophils was increased by 85% and 2 fold in S pneumoniae- and S aureus-induced pneumonia, respectively, compared with mice transfused with untreated neutrophils (Figure 6F). As a result, the mortality associated with neutropenia-related pneumonia also was drastically reduced (Figure 6G). Together, these findings further demonstrate that pretreatment of neutrophils with SF1670 before transfusion can significantly improve the efficacy of neutrophil transfusion.
Pretreatment with SF1670 also increases the efficacy of transfusion of G-CSF–mobilized neutrophils
Clinically, human neutrophils used in transfusion are collected from healthy donors who have been stimulated with G-CSF, which increases the abundance of circulating peripheral blood neutrophils by enhancing the proliferation of granulocytic precursors and promoting the mobilization of mature neutrophils. To mimic this clinical situation, we used a similar procedure to prepare G-CSF–mobilized mouse neutrophils for transfusion. As expected, the peripheral blood neutrophil count was increased dramatically after G-CSF treatment (Figure 7A). PTEN inhibitor SF1670 enhanced fMLP-induced PtdIns(3,4,5)P3 signaling in G-CSF–mobilized peripheral blood neutrophils (Figure 7B). Consistently, pretreatment with SF1670 also augmented fMLP-induced ROS production in these neutrophils (Figure 7C). In addition, similar with bone marrow derived mouse neutrophils, the efficacy of transfusion of G-CSF–mobilized neutrophils was significantly improved by SF1670 pretreatment (Figure 7D).
Pretreatment with SF1670 enhances the function and increases the efficacy of transfusion of G-CSF–mobilized neutrophils. (A) G-CSF (GCSF) induced neutrophil mobilization. A wild-type C57Bl/6 mouse was subcutaneously injected with G-CSF once daily at 250 μg/kg for 5 days. Peripheral blood (PB) was collected by cardiopuncture. White blood cells were counted using a Hematology Analyzer (Hemavet). (B) PTEN inhibitor SF1670 (SF) enhances fMLP-induced PtdIns(3,4,5)P3 signaling in both G-CSF–mobilized peripheral blood neutrophils and bone marrow–derived neutrophils (BM). Mouse peripheral blood samples were mixed with 2% dextran sulfate/PBS and 3 mg/mL EDTA/PBS at the ratio of 1:1:1 and incubated at 37°C for 20 minutes to allow red blood cells to settle. The leukocyte-containing layer was removed, and neutrophils were isolated by negative selection as described here. Isolated neutrophils were treated with SF1670 (500nM) or DMSO at 37°C for 30 minutes in PBS (0.2% bovine serum albumin), washed twice with HBSS, and then stimulated with 500nM fMLP for 4 minutes. The levels of phosphorylated AKT were assessed by Western blotting with specific antibody. (C) PTEN inhibitor SF1670 enhances fMLP-induced ROS production in G-CSF–mobilized peripheral blood neutrophils. Neutrophils were treated with SF1670 (500nM) or DMSO at 37°C for 30 minutes in PBS (0.2% BSA), washed twice with HBSS, and then stimulated with 2μM fMLP. ROS production was monitored in the presence of 50μM isoluminol and 0.8 U of horseradish peroxidase in a luminometer at 37°C. Chemiluminescence (arbitrary light units) was recorded at indicated time points. (D) Pretreatment with SF1670 increases the efficacy of transfusion of G-CSF–mobilized neutrophils. G-CSF–mobilized mouse peripheral blood neutrophils were pretreated with DMSO or SF1670 (250nM) for 30 minutes at 37°C, washed twice with PBS, and then intravenously injected into neutropenic mice that had been challenged with 105 CFU of E coli for 5 hours. Mice not receiving granulocyte transfusion were used as control. Histologic analysis of lungs reveals bacterial colonies (white arrow) in the pulmonary parenchyma. The number of bacterial colony was calculated. Pulmonary edema formation was quantified as the percentage of edema in the total parenchymal region using IPLab imaging software as described previously.29 . *P < .05, **P < .01 vs control; ##P < .01 vs DMSO.
Pretreatment with SF1670 enhances the function and increases the efficacy of transfusion of G-CSF–mobilized neutrophils. (A) G-CSF (GCSF) induced neutrophil mobilization. A wild-type C57Bl/6 mouse was subcutaneously injected with G-CSF once daily at 250 μg/kg for 5 days. Peripheral blood (PB) was collected by cardiopuncture. White blood cells were counted using a Hematology Analyzer (Hemavet). (B) PTEN inhibitor SF1670 (SF) enhances fMLP-induced PtdIns(3,4,5)P3 signaling in both G-CSF–mobilized peripheral blood neutrophils and bone marrow–derived neutrophils (BM). Mouse peripheral blood samples were mixed with 2% dextran sulfate/PBS and 3 mg/mL EDTA/PBS at the ratio of 1:1:1 and incubated at 37°C for 20 minutes to allow red blood cells to settle. The leukocyte-containing layer was removed, and neutrophils were isolated by negative selection as described here. Isolated neutrophils were treated with SF1670 (500nM) or DMSO at 37°C for 30 minutes in PBS (0.2% bovine serum albumin), washed twice with HBSS, and then stimulated with 500nM fMLP for 4 minutes. The levels of phosphorylated AKT were assessed by Western blotting with specific antibody. (C) PTEN inhibitor SF1670 enhances fMLP-induced ROS production in G-CSF–mobilized peripheral blood neutrophils. Neutrophils were treated with SF1670 (500nM) or DMSO at 37°C for 30 minutes in PBS (0.2% BSA), washed twice with HBSS, and then stimulated with 2μM fMLP. ROS production was monitored in the presence of 50μM isoluminol and 0.8 U of horseradish peroxidase in a luminometer at 37°C. Chemiluminescence (arbitrary light units) was recorded at indicated time points. (D) Pretreatment with SF1670 increases the efficacy of transfusion of G-CSF–mobilized neutrophils. G-CSF–mobilized mouse peripheral blood neutrophils were pretreated with DMSO or SF1670 (250nM) for 30 minutes at 37°C, washed twice with PBS, and then intravenously injected into neutropenic mice that had been challenged with 105 CFU of E coli for 5 hours. Mice not receiving granulocyte transfusion were used as control. Histologic analysis of lungs reveals bacterial colonies (white arrow) in the pulmonary parenchyma. The number of bacterial colony was calculated. Pulmonary edema formation was quantified as the percentage of edema in the total parenchymal region using IPLab imaging software as described previously.29 . *P < .05, **P < .01 vs control; ##P < .01 vs DMSO.
Discussion
Infection associated with therapy-related neutropenia continues to be a major cause of morbidity and mortality. Granulocyte transfusion therapy is principally used in the neutropenic patients to aid in the treatment of infections unresponsive to antibiotics and antifungal agents. Although controversy exists regarding the efficacy of current granulocyte transfusion practices, there is a general consensus regarding the need for further research in granulocyte biology to improve the function of transfused granulocytes in neutropenic patients with infections. Here, we show that the efficacy of granulocyte transfusion can be significantly improved using a specific PTEN inhibitor, SF1670. This will be an important and necessary complementation to the current antibiotic and G-CSF therapies. In addition, although we focus on neutropenia-related pneumonia in this study, the same strategy should be readily applied to other neutropenia-related infectious diseases.
The in vivo bacterial killing is a complex event that involves multiple cellular processes, such as neutrophil recruitment, superoxide production, and phagocytosis. In this study, we found that all 3 functions were augmented by pretreating neutrophils with SF1670. SF1670-treated neutrophils possess higher sensitivity to chemokine stimulation and elevated phagocytosis capability. Chemoattractant-induced transwell migration and reduced NADP oxidase-mediated superoxide production were augmented. In addition, the in vivo recruitment of transfused SF1670-pretreated neutrophils to the sites of inflammation was increased. Transfusion with SF1670-pretreated neutrophils also led to an augmentation of bacteria-killing capability (decreased bacterial burden) in the neutropenic recipient mice with both peritonitis and bacterial pneumonia. Consistently, this eventually alleviates the severity of and decreases the mortality of neutropenia-related pneumonia. Together, these observations provide direct evidence that augmenting PtdIns(3,4,5)P3 in transfused neutrophils with PTEN inhibitor SF1670 can boost innate immune responses and alleviate the severity of neutropenia-related infection.
Neutrophil functions also can be enhanced by priming, an important feature of the neutrophil inflammatory response.42-44 In this process, pretreatment with proinflammatory factors such lipopolysaccharide, G-CSF, platelet-activating factor, and tumour necrosis factor-α dramatically augments the functional response of neutrophils (eg, ROS production and degranulation) in response to subsequent stimuli such as C5a and fMLP. This phenomenon allows neutrophils to tightly control time and amplitude of superoxide production and degranulation, maximizing their role in the resolution of the inflammation, and minimizing the damage to surrounding tissue. However, no report in evidence indicates that priming also can elevate neutrophil recruitment and bacteria-killing capability. Thus, whether the efficacy of granulocyte transfusion can be improved by neutrophil priming needs to be further investigated. The priming effects are mediated by diverse cellular and biochemical mechanisms such as change in the level of cell-surface adhesion molecule expression, up-regulation of cell-surface receptors, production and release of bioactive lipids, up-regulation of calcium signaling, activation of signal transduction enzymes, and protein phosphorylation. It has been suggested that there may be multiple pathways leading to the same priming effect.44 However, unlike the priming agents, SF1670 enhances neutrophil function by specifically elevating PtdIns(3,4,5)P3 signaling. Consistent with the critical role of PtdIns(3,4,5)P3 signal in neutrophils, pretreatment with SF1670 can further enhance superoxide production in primed neutrophils (supplemental Figure 7). Thus, this observation further justifies SF1670 as a therapeutic target for improving the efficacy of current granulocyte transfusion practices.
The goal of this research is to enhance neutrophil function during granulocyte transfusion. Neutrophils are essential for host defense against invading pathogens. Augmentation of neutrophil function is beneficial for many pathologic conditions. However, hyperactivation of neutrophils also can lead to unwanted tissue damage and inflammation. Augmenting neutrophil function by elevating PtdIns(3,4,5)P3 signaling might increase the chance of more severe inflammation and tissue damage. However, this should be a less concern in neutropenic patients in which the number of neutrophils is dramatically reduced and the release of noxious compounds such as oxidants, proteases, and deoxyribonucleic acid by neutrophils is minimal. For example, in a mouse transfusion-related lung injury model, neutropenia significantly protects against lung injury.45
Another caveat of using PtdIns(3,4,5)P3 signaling activators, such as PTEN inhibitors, to improve granulocyte transfusion is that hyperactivation of PtdIns(3,4,5)P3 signaling also might induce cancer. Nevertheless, induction of cancer by elevating PtdIns(3,4,5)P3 signaling is a progressive process and usually takes several months or even years. In myeloid-specific PTEN knockout mice, we could not detect any tumor until 3 months after the birth.46 To improve the efficiency of granulocyte transfusion, PtdIns(3,4,5)P3 signal will only be elevated transiently; thus, it is unlikely that this type of treatment will lead to tumorigenesis. In addition, in the procedure described in current study, only the ex vivo-cultured neutrophils were treated with the PTEN inhibitor, and the neutrophils were washed before transfusion. Thus, in the recipients, only the transplanted neutrophils were exposed to the drugs. It is unlikely that leukemia will be developed from these terminally differentiated mature neutrophils. The prolonged intracellular retention of PTEN inhibitor SF1670 makes it an ideal candidate for specific inhibition of PTEN in transfused neutrophils.
In this study, we elevated PtdIns(3,4,5)P3 signaling by inhibiting lipid phosphatase PTEN. Other ways of elevating PtdIns(3,4,5)P3 signaling, such as suppressing Src homology 2-containing inositol 5′ phosphatase activity or enhancing phosphatidylinositol 3-kinase activity, also may improve the efficacy of granulocyte transfusion therapy. Consistently, enhanced recruitment of neutrophils in the lungs was detected in Src homology 2-containing inositol 5′ phosphatase-null neutrophils.47 In addition, the strength of PtdIns(3,4,5)P3 signaling can be regulated by mechanisms independently of its level in the plasma membrane. Activation of PtdIns(3,4,5)P3 signaling relies on PtdIns(3,4,5)P3-mediated plasma membrane translocation of PH domain-containing proteins, which was previously thought to be dependent solely on the concentration of PtdIns(3,4,5)P3 in the plasma membrane.20,24 Recently, we discovered that inositol phosphate (Ins)P7 and Ins(1,3,4,5)P4 compete with PtdIns(3,4,5)P3 for PH-domain binding and suppresses PH-domain translocation, providing a novel mode of regulation for PtdIns(3,4,5)P3 signaling.48,49 It will be intriguing to see whether the function of transfused neutrophils also can be enhanced by manipulating the intercellular levels of InsP7 and Ins(1,3,4,5)P4.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank John Manis, Li Chai, and John Luckey for helpful discussions and suggestions.
H.R.L. is supported by National Institutes of Health grants HL085100, AI076471, HL092020, and GM076084.
National Institutes of Health
Authorship
Contribution: Y.L. designed and performed experiments, collected, analyzed, and interpreted data, and prepared the manuscript; A.P. and Y.J. contributed to Figure 6; S.G.R. and P.K. contributed to Figure 7; F.L. and S.M. helped with data analysis; S.D. synthesized SF1670; L.E.S. designed experiments and analyzed data; and H.R.L. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hongbo R. Luo, Karp Family Research Bldg, Rm 10214, Boston, MA 02115; e-mail: hongbo.luo@childrens.harvard.edu.



![Figure 6. Pretreatment with SF1670 increases the efficacy of granulocyte transfusion in neutropenia-related pneumonia. (A) Mouse bone marrow–derived neutrophils were pretreated with or without SF1670 ([SF]; 250nM) for 30 minutes at 37°C, washed twice with PBS, and then intravenously injected to neutropenic mice that had been challenged with 105 CFU of E coli for 5 hours. Mice not receiving granulocyte transfusion were used as control. BALF was harvested 24 hours after each challenge. BALF was serially diluted and spread on plates. Colonies were counted next day. **P < .01 vs control; ##P < .01 vs DMSO. (B) Histologic analysis of lungs reveals bacterial colonies and polymerized fibrin in the pulmonary parenchyma (indicated by an arrow). Lung sections were stained with H&E. (C) Pulmonary edema formation was quantified as the percentage of edema in the total parenchymal region using IPLab imaging software as described previously.29 *P < .05, **P < .01 vs control; #P < .05 vs DMSO. (D) BALF total protein level. Protein accumulated in the inflamed lung was measure using a protein assay kit (Bio-Rad Laboratories). *P < .05, **P < .01 vs control; ##P < .01 vs DMSO. (E) Survival rates of mice infected with E coli. Age- and sex-matched neutropenia-related pneumonia mice were adoptively transferred with neutrophils pretreated with or without SF1670. Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. * P < .05 vs DMSO. (F) Pretreatment with SF1670 augments the bactericidal capability of the host in both S pneumoniae- and S aureus-induced neutropenia-related pneumonia. The experiments were conducted essentially as described in panel A. The pneumonia was induced by 106 CFU of S pneumonia or 107 CFU of S aureus. (G) Survival rates of mice infected with S aureus. The experiment was conducted essentially as described in panel E. *P < .05 vs DMSO-pretreated neutrophils.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-09-309864/4/m_zh89991173050006.jpeg?Expires=1766070258&Signature=y2MCNlujIWli0ICITUPNDG~bWMSQnaqcXp29dbFaizI5q3snQXcEhA1XI8TPPhCpd8iJ0k3FAx6LU5R~uBhI~H1Y18RNm6DItk8-Ip6julrf6xaJ8F9TAT~wPznR4uJGw1rKvdB1WGH7WSVSi-E22hqVBUwlCVnFVNRqMqACm7vrlLrr-5kVkbFDQO2OqWDqAcrcM-91oWeKRVYxu~V~3kezULuZF1SjL2XWtZg2xsHuECVNIWdSKbikMX6JxxI6Xtyn1fkNeI3~Q183Gpe2lZgO68dlAtG9y7cSCspG3dArHDILjLHLyYAME0OWBFUkQLBCnOfs84kgWUCF5O1-rA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 6. Pretreatment with SF1670 increases the efficacy of granulocyte transfusion in neutropenia-related pneumonia. (A) Mouse bone marrow–derived neutrophils were pretreated with or without SF1670 ([SF]; 250nM) for 30 minutes at 37°C, washed twice with PBS, and then intravenously injected to neutropenic mice that had been challenged with 105 CFU of E coli for 5 hours. Mice not receiving granulocyte transfusion were used as control. BALF was harvested 24 hours after each challenge. BALF was serially diluted and spread on plates. Colonies were counted next day. **P < .01 vs control; ##P < .01 vs DMSO. (B) Histologic analysis of lungs reveals bacterial colonies and polymerized fibrin in the pulmonary parenchyma (indicated by an arrow). Lung sections were stained with H&E. (C) Pulmonary edema formation was quantified as the percentage of edema in the total parenchymal region using IPLab imaging software as described previously.29 *P < .05, **P < .01 vs control; #P < .05 vs DMSO. (D) BALF total protein level. Protein accumulated in the inflamed lung was measure using a protein assay kit (Bio-Rad Laboratories). *P < .05, **P < .01 vs control; ##P < .01 vs DMSO. (E) Survival rates of mice infected with E coli. Age- and sex-matched neutropenia-related pneumonia mice were adoptively transferred with neutrophils pretreated with or without SF1670. Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. * P < .05 vs DMSO. (F) Pretreatment with SF1670 augments the bactericidal capability of the host in both S pneumoniae- and S aureus-induced neutropenia-related pneumonia. The experiments were conducted essentially as described in panel A. The pneumonia was induced by 106 CFU of S pneumonia or 107 CFU of S aureus. (G) Survival rates of mice infected with S aureus. The experiment was conducted essentially as described in panel E. *P < .05 vs DMSO-pretreated neutrophils.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-09-309864/4/m_zh89991173050006.jpeg?Expires=1766266354&Signature=WRohHIoh9G8uoHdFEKzM405haSYzTc~4w87~HynKu6Xb3taHN7nypA4opZ2~fgC-bFM28aWJIIzm8vDK4Lj5c8pEhxik2Q9CMXjS~GSnsPxLn5hJpW2fLQ4J5Qo2FhUF9QAjx4gklcYs39-~SdAREtdkZwwl2qw3kQvdPWDTqyb-wZIALLwtmSTyBDzFN079-4qogcFxj5fOvAF8qYLL3H4PLGjYHvimDg3WLdn6Z3y66yC2T0NJOVFWlIedcxu2G~BDoN2rbc-AUmJxxpzMIRHNzbkbE-WNdWKvRBJ8s98dD3qXqn5JKhMEnkC41wxKMNrWIcLAnhRdTNaTMUrqZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
