Abstract
Blocking heat-shock protein 90 (Hsp90) induces death of malignant plasma cells by activation of the unfolded protein response, a signaling pathway activated by accumulation of misfolded proteins within the endoplasmic reticulum. We hypothesized that nontransformed plasma cells are also hypersensitive to Hsp90 inhibition because of their high amount of protein biosynthesis. To investigate this hypothesis, 2 different Hsp90 inhibitors, the geldanamycin derivative 17-DMAG and the nontoxic peptide derivative TCBL-145, were applied to mice with experimental epidermolysis bullosa acquisita, an autoimmune bullous disease characterized by autoantibodies against type VII collagen of the dermal-epidermal junction. Both inhibitors ameliorated clinical disease of type VII collagen–immunized mice, suppressed auto-antibody production, and reduced dermal neutrophilic infiltrate. Interestingly, total plasma cell numbers, type VII collagen–specific plasma cells, and germinal center B cells were unaffected by anti-Hsp90 treatment in vivo. However, T-cell proliferation was potently inhibited, as evidenced by the reduced response of isolated lymph node cells from immunized mice to in vitro restimulation with anti-CD3/CD28 antibody or autoantigen in the presence of Hsp90 inhibitors. Our results suggest that Hsp90 blockade has no impact on normal or autoreactive plasma cells in vivo and indentify T cells as targets of anti-Hsp90 treatment in autoimmunity to type VII collagen.
Introduction
Autoreactive T cells, B cells, and plasma cells have been identified as key players in the pathophysiology of autoimmune diseases. Although much progress has been made in revealing the immunologic processes in these diseases, their therapy remains challenging and in most cases still consists of conventional, unspecific immunosuppressive treatment with corticosteroids and cytostatic agents. However, the application of these drugs is often limited due to side effects, and disease remission frequently cannot be achieved.1
Epidermolysis bullosa acquisita (EBA) is a chronic subepidermal blistering disease characterized by circulating and tissue-bound autoantibodies targeting the noncollagenous domain 1 of type VII collagen, a major component of anchoring fibrils of the dermal-epidermal junction.2,3 The pathogenic relevance of (auto)antibodies against type VII collagen has been conclusively shown ex vivo and in experimental animal models.4 In EBA and most other subepidermal autoimmune bullous diseases, autoantibodies alone are not sufficient to induce blisters, but require an Fc-dependent engagement of humoral and cellular inflammatory factors.4 In addition, a key role of T cells in the initiation of autoimmunity against type VII collagen has been demonstrated recently.5 Experimental EBA, which reproduces both the autoimmune response and immunopathologic, histologic, and clinical findings in patients with EBA, can be induced in susceptible mice by immunization with recombinant murine type VII collagen.6 Therefore, EBA emerges as a model disease to study fundamental biologically and clinically crucial aspects of antibody-mediated, organ-specific autoimmune diseases.
Heat-shock proteins (Hsps) are molecular chaperones essential for maintaining cellular functions by preventing misfolding and aggregation of nascent polypeptides and by facilitating protein folding.7 Pharmacologic inhibition of the Hsp family member Hsp90 has been primarily implicated in the context of cancer treatment because this chaperone is used by many cancer cells to facilitate the function of numerous oncoproteins.8 Several Hsp90 inhibitors with different side-effect profiles have been identified,9,10 of which newly synthesized, short-peptide derivatives (eg, TCBL-145) are of particular interest because in vivo toxicity has so far not been reported.10
Recently, Hsp90 has been reported to play important roles in antigen presentation, activation of lymphocytes and macrophages, and activation and maturation of dendritic cells, indicating a potential treatment target of inflammatory diseases, including autoimmune diseases.11 Indeed, pharmacologic inhibition of Hsp90 has recently been successfully applied in mouse models of autoimmune encephalomyelitis,12 rheumatoid arthritis,13 and systemic lupus erythematosus–like autoimmune disease.14
Although the inhibitory effects of Hsp90 inhibitors on various immune cells, including T cells, have been reported in autoimmunity,12-14 the direct impact of anti-Hsp90 treatment on autoantibody-producing plasma cells has not been studied in vivo. Plasma cells producing high levels of protein (ie, immunoglobulin) are dependent on the unfolded protein response (UPR), which maintains protein homeostasis within the endoplasmic reticulum (ER) to ensure cell survival.15 Activation of the UPR results in a bias of translation toward the synthesis of chaperone proteins involved in protein folding, an increase in the degradation of misfolded proteins via the ubiquitin proteasome pathway, and the delivery of a survival signal. If these objectives are not achieved within a certain time frame or if the disruption is prolonged, an ER stress signal is generated and apoptosis ensues.16 It has been shown that the proteasome inhibitor bortezomib can induce a UPR, leading to apoptosis of malignant plasma cells in vitro.17 Hsp90b1, the ER paralog of the cytosolic Hsp90, is believed to be one of the key molecular chaperones controlling the UPR.16 Similar to proteasome inhibition, blockade of Hsp90 has been associated with activation of the UPR pathway and apoptosis caused by an overload of unfolded monoclonal paraproteins in myeloma plasma cell lines.18 Recently, Neubert et al19 reported that bortezomib is also capable of depleting normal and autoreactive plasma cells by activation of the UPR and of protecting mice from lupus-like disease. We therefore hypothesized that, similar to blocking the disposal of misfolded immunoglobulins by bortezomib, disrupting client-chaperone interactions using Hsp90 inhibitors would result in an inability to handle immunoglobulin production and death of not only malignant but also autoreactive plasma cells.
In the present study, we show that pharmacologic blockade of Hsp90 by the geldanamycin derivative 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) or the nontoxic peptide derivative TCBL-145 is associated with amelioration of type VII collagen–induced EBA in mice, and that T cells, rather than plasma cells, represent targets of anti-Hsp90 treatment in this autoimmune disease.
Methods
Mice
Six- to 8-week-old SJL mice were purchased from Charles River Laboratories. The experiments were approved by local authorities of the Animal Care and Use Committee (Kiel, Germany; reference number: 6/1f/08) and performed by certified personnel.
Production of autoantigen
The recombinant fragment of murine type VII collagen (mCVIIC; amino acids 757-967) was prepared as described previously.20-22 Recombinant tagged fragments GST-mCVIIC and His-mCVIIC were produced using a prokaryotic expression system and purified by glutathione and metallochelate affinity chromatography, respectively.20,22
Induction of EBA and phenotype analysis
Experimental EBA was induced in mice by active immunization, as described previously.22 Briefly, mice were injected subcutaneously in the hind footpads with a single injection of 100 μL of emulsion containing 60 μg of GST-mCVIIC in TiterMax (Alexis Biochemicals). Mice were examined every week for their general condition and for cutaneous lesions (ie, erythema, blisters, erosions, and crusts). Disease severity was calculated as the percentage of the body surface area affected by skin lesions.
Treatment of mice
We treated mice intraperitoneally with 30 mg/kg of 17-DMAG (InvivoGen) or 3 mg/kg of TCBL-145 (produced by the Medical Chemistry Group at University of Szeged, Szeged, Hungary). We injected control mice with an equivalent volume of the solvent that is, water or water with 5% ethanol, respectively. For prophylactic treatment, mice received a total of 2 injections with 17-DMAG or vehicle 1 day before and 1 day after immunization or with a total of 14 daily injections with TCBL-145 or vehicle starting 1 day before immunization, and were followed for 6 weeks. In a second experimental paradigm, if > 2% of the body surface area was affected by EBA lesions, we analyzed the therapeutic effect of 17-DMAG compared with vehicle 3 times a week or TCBL-145 compared with vehicle once a day, each given over a 6-week treatment period. One day after the last injection, mice were killed for clinical and immunologic evaluation. In a third set of experiments, we gave immunized mice 17-DMAG or vehicle twice with an interval of 36 hours between injections. We analyzed mice 48 hours after the first injection.
Histopathology and immunofluorescence microscopy
Biopsies of lesional and peri-lesional skin were prepared for examination by histopathology and immunofluorescence microscopy, as described previously.20 Briefly, biopsies collected from experimental animals were fixed in 4% buffered formalin, and sections from paraffin-embedded tissues were stained with hematoxylin and eosin. Dermal neutrophil infiltration was assessed semiquantitatively using the following scoring system: infiltrated 0 = no infiltration, 1 = faint infiltration, 2 = moderate infiltration, and 3 = severe infiltration. IgG and C3 deposits were detected by direct immunofluorescence microscopy on frozen sections prepared from tissue biopsies using 100-fold diluted FITC–labeled antibodies specific to mouse IgG (Dako) and murine C3 (MP Biomedicals). The staining intensity of immunoreactants in the skin of mice was quantified with ImageJ software. Sera were assayed for antibody titers by indirect immunofluorescence microscopy on cryosections of normal mouse skin using diluted mouse serum (1:10-1:20.000) and 100-fold diluted FITC labeled antibodies specific to mouse IgG (Dako).
Detection of plasma and germinal center B cells by flow cytometry
Single-cell suspensions were prepared from bone marrow (from femurs) and spleens, as described previously.23 To detect plasma cells, flow cytometric analyses of splenocytes and bone marrow cells were performed using fluorochrome-conjugated monoclonal antibodies to mouse CD45R (B220, clone RA3.6B2; made in-house) and CD138 (BD Biosciences), as described previously.19 For analysis of germinal center B cells, peanut hemagglutinin (PNA; Vector Laboratories) was used. Samples were analyzed on an LSR II flow cytometer (BD Biosciences).
Detection of autoreactive type VII collagen–specific plasma cells by immunohistochemistry
Cryostat sections of draining lymph nodes were fixed in methanol-acetone, incubated with biotinylated recombinant His-mCVIIC, and stained with ExtrAvidin-alkaline phosphatase and FastBlue BB salt (Sigma-Aldrich). The sections were then incubated with rat anti–mouse CD138 (BD Pharmingen) and counterstained with peroxidase-conjugated rabbit anti–rat IgG (H+L; Jackson ImmunoResearch Laboratories) and DAB (DakoCytomation). Before further analysis, immunohistochemical double staining of mCVIIC-specific CD138+ cells was confirmed by immunofluorescence staining (data not shown). mCVIIC-specific CD138+ cells were counted in representative slices of each draining lymph node, and numbers were related to the measured area of the respective slice using the PALM MicroBeam system (Zeiss).
Assessment of T-cell proliferation
The T-cell proliferation assay was performed as described previously with minor modifications.5 Single-cell suspensions of draining lymph nodes of immunized mice were cultured in RPMI 1640 culture medium supplemented with 1mM sodium pyruvate, 2mM glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, nonessential amino acids, 50μM β-mercaptoethanol, and 10% FCS. A total of 2.5 × 105 cells were cultured in the presence of either tissue culture plate–bound anti-CD3 mAbs (2.5 μg/mL; BD Biosciences) with soluble anti-CD28 mAbs (1 μg/mL; BD-Biosciences) or 50 μg/mL of autoantigen (His-mCVIIC) in 96-well plates. Stimulated cells were cultured alone or with different concentrations of 17-DMAG (0.1, 0.25, or 2.5μM) or TCBL-145 (25, 50, or 75μM). After 96 hours, cells were pulsed with BrdU for a further 24 hours. Cell proliferation was measured using a colorimetric cell-proliferation BrdU ELISA (Roche). Cell viability was evaluated by trypan blue exclusion.
Statistical analyses
All analyses were performed using t test or ANOVA (Bonferroni procedure for multiple comparisons). Data are presented as means ± SEM; P < .05 was considered statistically significant.
Results
Prophylactic effects of Hsp90 inhibitors on type VII collagen–induced EBA
We tested the effects of early administration of 17-DMAG and TCBL-145 on the development of clinical symptoms in mice immunized with GST-mCVIIC. In this EBA model, SJL mice typically show a disease incidence of > 90%, with clinical signs as early as 2 weeks after immunization and clinical disease maintenance for at least 24 weeks.4,6 When administered 1 day before and 1 day after (17-DMAG) or for 14 days starting 1 day before immunization (TCBL-145), both Hsp90 inhibitors significantly reduced disease development in mice (Figure 1A-B). The maximum individual scores, defined as the percentage of body surface area affected by skin lesions, were 13 in the vehicle-treated mice and 3 in the Hsp90-treated mice when analyzed 6 weeks after immunization. While scores were always > 1 and disease incidence was 100% in both vehicle groups, 75% of 17-DMAG–treated mice had scores < 1 and 67% of mice treated with TCBL-145 were completely free of clinical signs.
Early administration of Hsp90 inhibitors suppresses type VII collagen–induced development of EBA clinical phenotype. (A) Vehicle or 17-DMAG (30 mg/kg) was administered 1 day before and 1 day after immunization of SJL mice with 60 μg of GST-mCVIIC. (B) Vehicle or TCBL-145 (3 mg/kg) was given daily for 14 days starting 1 day before immunization. Data are presented as the average clinical scores defined as the percentage of body surface area affected by skin lesions 6 weeks after immunization. Values are means ± SEM of ≥ 6 mice per group. *P < .05.
Early administration of Hsp90 inhibitors suppresses type VII collagen–induced development of EBA clinical phenotype. (A) Vehicle or 17-DMAG (30 mg/kg) was administered 1 day before and 1 day after immunization of SJL mice with 60 μg of GST-mCVIIC. (B) Vehicle or TCBL-145 (3 mg/kg) was given daily for 14 days starting 1 day before immunization. Data are presented as the average clinical scores defined as the percentage of body surface area affected by skin lesions 6 weeks after immunization. Values are means ± SEM of ≥ 6 mice per group. *P < .05.
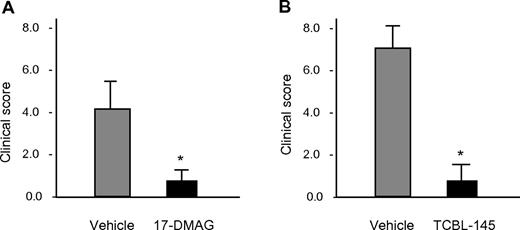
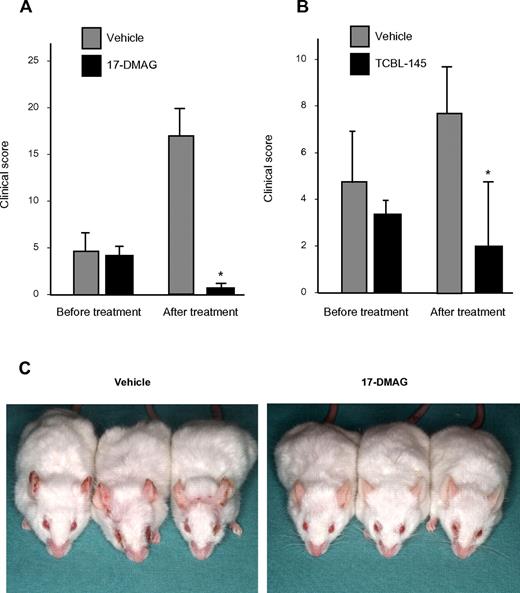
Therapeutic effects of Hsp90 inhibitors on type VII collagen–induced EBA
To investigate the therapeutic efficacy of Hsp90 inhibitors in EBA and to study their effects on immunologic parameters of this disease, 17-DMAG and TCBL-145 were given to mice during ongoing disease. Immunized mice were allowed to reach moderate clinical severity (primarily individual scores of ≥ 2), and were then administered 17-DMAG or TCBL-145 for 6 weeks. While disease activity progressed in the vehicle-treated groups, the average clinical score decreased significantly from 4.1 ± 0.6 to 0.9 ± 0.3 in the 17-DMAG–injected mice and from 3.3 ± 0.4 to 1.7 ± 0.8 in the TCBL-145–treated mice by the end of the study (Figure 2A-C). No signs of toxicity, weight loss, or mortality were found during treatment with either Hsp90 inhibitor.
Hsp90 inhibitors exert therapeutic activity in type VII collagen–induced EBA. Mice were immunized with 60 μg of GST-mCVIIC and disease was allowed to progress until moderate average clinical scores (primarily ≥ 2) were observed, at which time vehicle vs 17-DMAG (30 mg/kg 3 times a week; A) or vehicle vs TCBL-145 (3 mg/kg daily; B) was administered over a 6-week treatment period. Data are presented as the average clinical scores defined as the percentage of body surface area affected by skin lesions at the end of the treatment period. Values are means ± SEM of ≥ 6 mice per group. *P < .05. (C) Representative clinical presentations of vehicle- and 17-DMAG–treated mice at the end of the treatment period. Vehicle-treated mice had erythema, erosions, and crusts predominantly located on the ears and around the eyes, whereas 17-DMAG–injected mice presented with significantly less severe disease.
Hsp90 inhibitors exert therapeutic activity in type VII collagen–induced EBA. Mice were immunized with 60 μg of GST-mCVIIC and disease was allowed to progress until moderate average clinical scores (primarily ≥ 2) were observed, at which time vehicle vs 17-DMAG (30 mg/kg 3 times a week; A) or vehicle vs TCBL-145 (3 mg/kg daily; B) was administered over a 6-week treatment period. Data are presented as the average clinical scores defined as the percentage of body surface area affected by skin lesions at the end of the treatment period. Values are means ± SEM of ≥ 6 mice per group. *P < .05. (C) Representative clinical presentations of vehicle- and 17-DMAG–treated mice at the end of the treatment period. Vehicle-treated mice had erythema, erosions, and crusts predominantly located on the ears and around the eyes, whereas 17-DMAG–injected mice presented with significantly less severe disease.
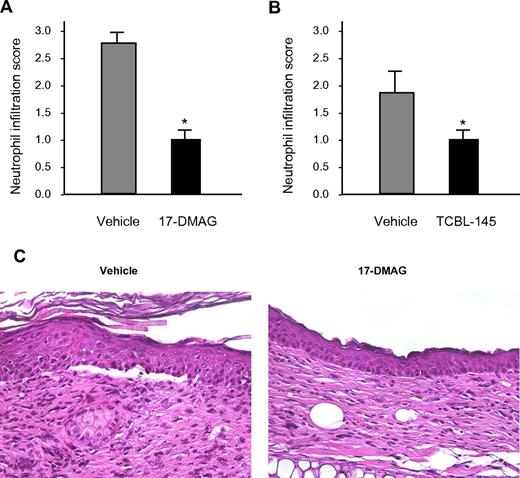
Histologic examination of lesional skin biopsies from vehicle-treated mice revealed characteristic dermal-epidermal separation accompanied by dense inflammatory infiltrates that were dominated by neutrophils. Dermal neutrophil infiltration was significantly reduced in 17-DMAG- and TCBL-145–treated mice compared with vehicle-injected animals (Figure 3A-C).
Hsp90 inhibitors reduce dermal neutrophil infiltration. Semiquantitative scoring of dermal neutrophil infiltration ranging from 0 (no infiltration) to 3 (severe infiltration) in mice treated with vehicle versus 17-DMAG (A) or vehicle vs TCBL-145 (B) at the end of the 6-week treatment period. Values are means ± SEM of ≥ 6 mice per group. *P < .05. (C) Representative histopathology specimens obtained from the ears of mice injected with vehicle or 17-DMAG by the end of the treatment period. In contrast to 17-DMAG–treated mice, vehicle-injected mice showed dermal-epidermal separation and stronger dermal inflammatory infiltrate dominated by neutrophils (magnification, 400×).
Hsp90 inhibitors reduce dermal neutrophil infiltration. Semiquantitative scoring of dermal neutrophil infiltration ranging from 0 (no infiltration) to 3 (severe infiltration) in mice treated with vehicle versus 17-DMAG (A) or vehicle vs TCBL-145 (B) at the end of the 6-week treatment period. Values are means ± SEM of ≥ 6 mice per group. *P < .05. (C) Representative histopathology specimens obtained from the ears of mice injected with vehicle or 17-DMAG by the end of the treatment period. In contrast to 17-DMAG–treated mice, vehicle-injected mice showed dermal-epidermal separation and stronger dermal inflammatory infiltrate dominated by neutrophils (magnification, 400×).
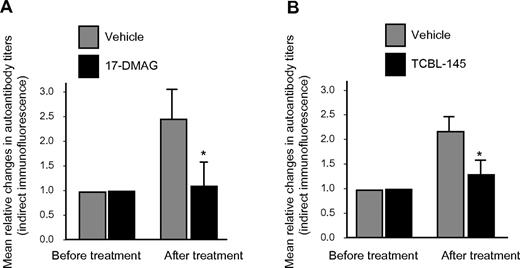
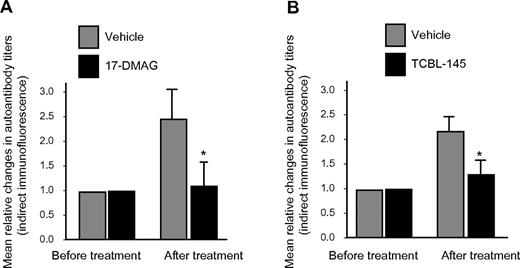
The generation of circulating IgG autoantibodies against the dermal-epidermal junction was sufficiently suppressed by 17-DMAG or TCBL-145, resulting in significantly lower serum autoantibody levels compared with vehicle-injected animals at the end of the observation period, as detected by indirect immunofluorescence microscopy (Figure 4A-B). However, no substantial differences in signal intensities for basal membrane–bound anti–type VII collagen IgG or C3 were found by direct immunofluorescence microscopy between the treatment groups (data not shown).
Hsp90 inhibitors suppress autoantibody production. Mean relative titer changes of autoantibodies directed against the dermal-epidermal junction as detected by indirect immunofluorescence microscopy using normal murine skin as a substrate after the 6-week treatment period with vehicle versus 17-DMAG (A) or vehicle vs TCBL-145 (B). Values are means ± SEM of ≥ 6 mice per group. *P < .05.
Hsp90 inhibitors suppress autoantibody production. Mean relative titer changes of autoantibodies directed against the dermal-epidermal junction as detected by indirect immunofluorescence microscopy using normal murine skin as a substrate after the 6-week treatment period with vehicle versus 17-DMAG (A) or vehicle vs TCBL-145 (B). Values are means ± SEM of ≥ 6 mice per group. *P < .05.
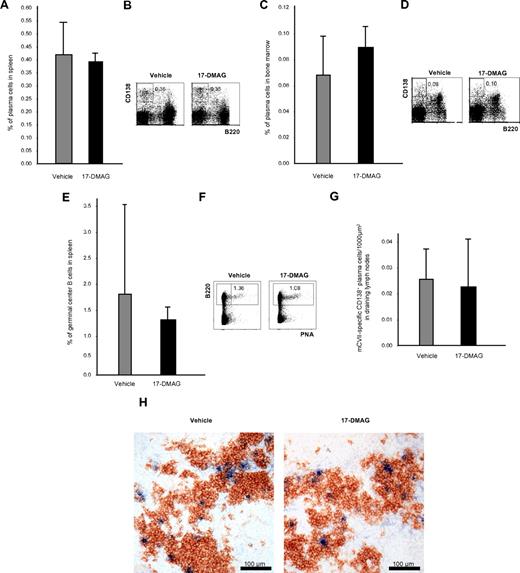
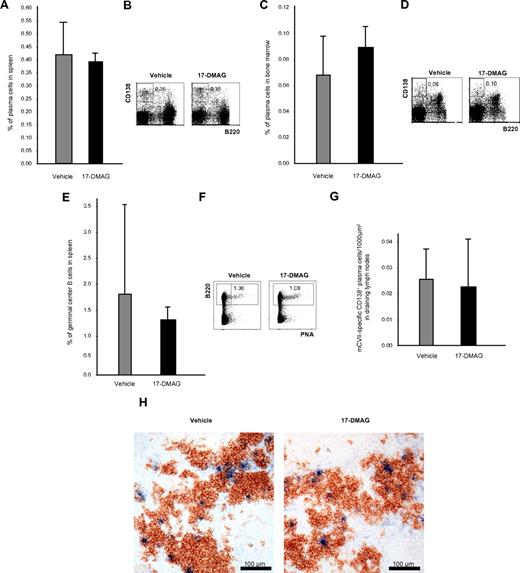
Effects of Hsp90 inhibitors on plasma and germinal center B cells
To investigate whether Hsp90 inhibitors eliminate not only malignant but also normal plasma cells, B220−CD138+ plasma cells from spleen and bone marrow were investigated by flow cytometry 24 hours after the last injection of 17-DMAG and TCBL-145 given over 6 weeks. Cytofluorimetric quantification revealed no difference in the number of splenic or bone marrow plasma cells in vehicle- and 17-DMAG–treated mice (Figure 5A-D). B220+PNA+ germinal center B cells in the spleen were also not significantly affected (Figure 5E-F). To rule out compensatory mechanisms of plasma cells after long-term treatment with Hsp90 inhibitors, splenic plasma cells were also analyzed 48 hours after the start of treatment with 17-DMAG. No depletion of plasma cells was achieved after short-term treatment (data not shown). Comparable results were obtained with TCBL-145 (data not shown).
Plasma cells are not affected by Hsp90 inhibitors in vivo. (A) Bar graph of flow cytometric analysis of the frequency of B220−CD138+ plasma cells in the spleen after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (B) Representative corresponding flow cytometric analysis, with numbers in the top panels representing the percentages of plasma cells with respect to total cell numbers. (C) Bar graph of flow cytometric analysis of the frequency of B220−CD138+ plasma cells in the bone marrow after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (D) Representative corresponding flow cytometric analysis, with numbers in the top panels representing the percentages of plasma cells with respect to total cell numbers. (E) Bar graph of flow cytometric analysis of the frequency of B220+PNA+ germinal center B cells in the spleen after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (F) Representative corresponding flow cytometric analysis, with numbers in the top panels representing percentages of plasma cells with respect to total cell numbers. (G) Bar graph of immunohistochemical analysis of the frequency of CD138+ mCVIIC-specific autoreactive plasma cells from draining lymph nodes after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (H) Representative immunohistochemical specimens obtained from draining lymph nodes of mice injected with vehicle or 17-DMAG illustrating comparable numbers of autoreactive plasma cells (double stained in blue and brown) by the end of the treatment period.
Plasma cells are not affected by Hsp90 inhibitors in vivo. (A) Bar graph of flow cytometric analysis of the frequency of B220−CD138+ plasma cells in the spleen after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (B) Representative corresponding flow cytometric analysis, with numbers in the top panels representing the percentages of plasma cells with respect to total cell numbers. (C) Bar graph of flow cytometric analysis of the frequency of B220−CD138+ plasma cells in the bone marrow after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (D) Representative corresponding flow cytometric analysis, with numbers in the top panels representing the percentages of plasma cells with respect to total cell numbers. (E) Bar graph of flow cytometric analysis of the frequency of B220+PNA+ germinal center B cells in the spleen after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (F) Representative corresponding flow cytometric analysis, with numbers in the top panels representing percentages of plasma cells with respect to total cell numbers. (G) Bar graph of immunohistochemical analysis of the frequency of CD138+ mCVIIC-specific autoreactive plasma cells from draining lymph nodes after 6 weeks of treatment with vehicle or 17-DMAG. Values are means ± SEM of ≥ 6 mice per group. P > .05. (H) Representative immunohistochemical specimens obtained from draining lymph nodes of mice injected with vehicle or 17-DMAG illustrating comparable numbers of autoreactive plasma cells (double stained in blue and brown) by the end of the treatment period.
We also investigated whether Hsp90 inhibitors may have an impact on autoreactive plasma cells. Similar to normal plasma cells, immunohistochemical studies revealed that the numbers of CD138+ mCVIIC-specific plasma cells from draining lymph nodes were not significantly altered by 17-DMAG after long-term (6 weeks) (Figure 5G-H) or short-term treatment (48 hours) or treatment with TCBL-145 (data not shown).
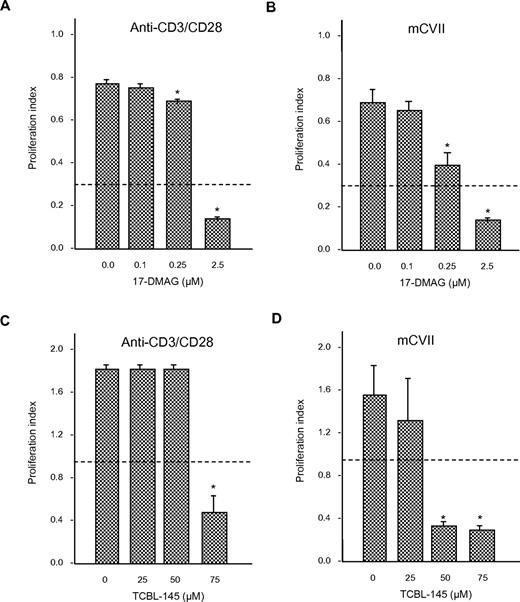
Effects of Hsp90 inhibitors on T-cell proliferation
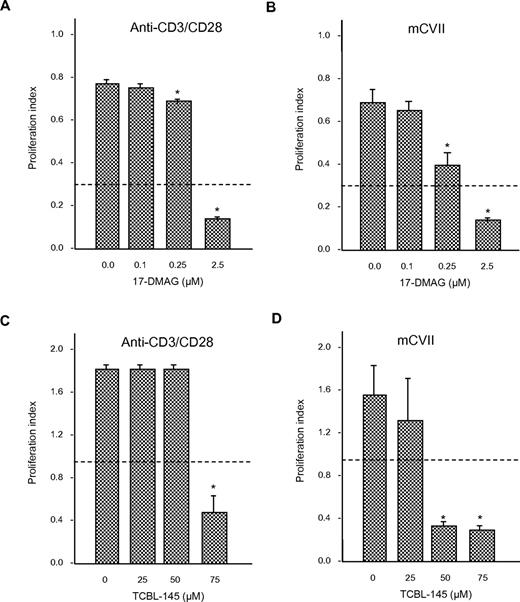
To determine whether 17-DMAG or TCBL-145 affected T-cell proliferation, we isolated draining lymph node cells from mice 6-8 weeks after immunization with GST-mCVIIC and stimulated them with anti-CD3/CD28 antibody or autoantigen in the absence or presence of different amounts of 17-DMAG or TCBL-145. Cells from immunized mice proliferated in response to ex vivo activation with the stimulants. The addition of 17-DMAG or TCBL-145 significantly abolished (autoreactive) T-cell proliferation in a dose-dependent manner (Figure 6A-D). Cell viability after treatment with either Hsp90 inhibitor was > 80%, as measured by the amount of trypan blue–negative (ie, living) cells.
Anti-Hsp90 treatment leads to inhibition of T-cell proliferation ex vivo. Draining lymph node cells were isolated from GST-mCVIIC–immunized mice 6-8 weeks after immunization and reactivated with anti-CD3 (2.5 μg/mL) and anti-CD28 (1 μg/mL) antibody, or the recombinant autoantigen His-mCVIIC (50 μg/mL). Incubations were performed in the absence or presence of different amounts of 17-DMAG (A-B) or TCBL-145 (C-D), and T-cell proliferation was determined by BrdU ELISA. Dotted lines represent the values obtained from cells incubated with medium alone. Values are means ± SEM of 6 mice per group. *P < .05.
Anti-Hsp90 treatment leads to inhibition of T-cell proliferation ex vivo. Draining lymph node cells were isolated from GST-mCVIIC–immunized mice 6-8 weeks after immunization and reactivated with anti-CD3 (2.5 μg/mL) and anti-CD28 (1 μg/mL) antibody, or the recombinant autoantigen His-mCVIIC (50 μg/mL). Incubations were performed in the absence or presence of different amounts of 17-DMAG (A-B) or TCBL-145 (C-D), and T-cell proliferation was determined by BrdU ELISA. Dotted lines represent the values obtained from cells incubated with medium alone. Values are means ± SEM of 6 mice per group. *P < .05.
Discussion
The results of the present study indicate that in contrast to malignant plasma cells, normal or autoreactive plasma cells do not present targets of Hsp90 inhibitors in vivo, and that the efficacy of anti-Hsp90 treatment is at least in part mediated by immunosuppressive functions on T-cell responses in autoimmunity to type VII collagen.
17-DMAG and TCBL-145 effectively suppressed the development of EBA disease when administered before the appearance of clinical signs and induced clinical recovery when applied to mice that were already diseased. Compared with control mice, animals treated with Hsp90 inhibitors showed a reduced dermal inflammatory infiltrate at the dermal-epidermal junction and lower levels of circulating autoantibodies against the basal membrane zone. Our data are in agreement with previous observations in animal models of other autoimmune diseases, including autoimmune encephalomyelitis,12 rheumatoid arthritis,13 and systemic lupus erythematosus–like autoimmune disease,14 in which inhibitors of Hsp90 affected inflammatory disease pathways and efficiently improved the clinical course. The mechanisms of action by which Hsp90 inhibitors led to clinical improvement in these mouse models included reductions in: (1) maturation of dendritic cells,14 (2) populations of antigen-presenting cells,14 (3) activated T and B cells,12,14 and (4) cytokine production.12,13 However, the effects of anti-Hsp90 treatment on autoantibody-producing plasma cells have not yet been studied. This is an important issue to address because Hsp90b1 is believed to be one of the key downstream chaperones in the ER that controls the ER UPR, which is increasingly being shown to modulate plasma cell function.15
In vitro, both proteasome and Hsp90 inhibition have been linked to UPR-mediated death of malignant plasma cells in multiple myeloma caused by the buildup of misfolded immunoglobulins within the ER.17,18 It was later shown that normal plasma cells are also hypersensitive to proteasome inhibition because of their extremely high amount of protein biosynthesis. Bortezomib depletes normal and autoimmune plasma cells from bone marrow and spleen in vivo via activation of the UPR and protects mice with lupus-like disease from nephritis.19 In contrast, we found no effect of anti-Hsp90 treatment on the survival of normal or autoreactive plasma cells in vivo. The numbers of B220−CD138+ plasma cells from spleen and bone marrow and type VII collagen–specific plasma cells from draining lymph nodes after the 6-week treatment with either 17-DMAG or TCBL-145 were comparable to plasma cell counts of mice treated with vehicle. Splenic plasma cells were also not depleted after a 48-hour short-term treatment with 17-DMAG, ruling out possible compensatory mechanisms that may have occurred during long-term treatment. Germinal center B cells were also not affected by Hsp90 inhibitors in vivo.
In contrast to the findings obtained with Hsp90 inhibition in transformed plasma cells,18 our data imply that in vivo anti-Hsp90 treatment does not lead to sufficient ER stress for initiation of the UPR and subsequent apoptosis of nonmalignant plasma cells. It is possible that Hsp90 exhibits diverse susceptibility toward its inhibitors in malignant and nonmalignant plasma cells. In fact, previous studies have established that Hsp90 produced by cancer cells is found within a multichaperone complex associated with high ATPase activity, which has a 100-fold higher affinity for inhibitors than its free, uncomplexed form expressed in normal cells.24 Another assumption is that either immunoglobulin is not the true client protein of Hsp90b1 or the immunoglobulin-chaperoning function is redundant and can be compensated for by other immunoglobulin-interacting ER chaperones such as GRP7825 and GRP170.26 A recent study found no intrinsic defect with B cells from B-cell–specific Hsp90b1-null mice in terms of B-cell receptor expression, proximal signaling, immunoglobulin assembly and production, and plasma cell differentiation.27 Our data extend these previous observations and indicate that targeting Hsp90 has no impact on the survival of normal or autoreactive plasma cells in vivo.
Because T cells have recently been found to be required for both the production of autoantibodies and blistering in experimental EBA,5 we investigated whether these cells are affected by Hsp90 inhibition. Isolated draining lymph node cells from immunized mice stimulated with the T-cell–activating anti-CD3/CD28 antibody revealed a dose-dependent reduction of proliferation index when cocultured with Hsp90 inhibitors. Restimulation with recombinant type VII collagen was also suppressed, indicating that autoreactive T cells were targets of anti-Hsp90 treatment. These findings are in agreement with previous studies showing an inhibitory potential of Hsp90 blockade on activated T cells,12,14,28,29 including those of the autoreactive12,14 and alloreactive type.29
Although in the present study, autoreactive plasma cells were not depleted by anti-Hsp90 treatment, we found a suppressed serum autoantibody production in anti-Hsp90–treated mice. There are 2 mutually nonexclusive explanations for this observation. The first is linked to the inhibitory effects of Hsp90 inhibitors on T cells, which are known to provide help to B cells. The second is based on the observation that the generation of a T cell–dependent, antigen-specific antibody response requires activation of Toll-like receptors in B cells, and on the increasing appreciation of the importance of these receptors in the pathophysiology of autoimmune diseases.30,31 Because HSP90b1 ablation in B cells has been recently shown to be associated with an attenuated antibody production in the context of Toll-like receptor stimulation,27 disruption of chaperoning Toll-like receptors rather than immunoglobulin assembly might have accounted for this suppressed autoantibody response after Hsp90 treatment.
Presumably, the clinical effects of Hsp90 inhibitors observed in our experiments do not exclusively depend on the inhibition of T cells. Inhibitory effects on dendritic cells might have also played a role, because there is increasing evidence of Hsp90 inhibitors interfering with dendritic cell function and autoantigen presentation.11,14 Neutrophils, proinflammatory cytokines, and proteases, all of which are important pathogenic factors in EBA,4 might have been additional targets. In fact, we found reduced numbers of neutrophils in the skin after anti-Hsp90 treatment compared with control mice.
In conclusion, our results suggest that in contrast to killing malignant plasma cells, Hsp90 inhibitors do not affect normal or autoimmune plasma cells in vivo, but do exhibit suppressive effects on T-cell function. Therefore, the therapeutic approach of selectively depleting autoimmune plasma cells remains confined to the proteasome inhibitor bortezomib, although its clinical use may be limited because of significant toxicity.32 The strong in vivo efficacy observed with anti-Hsp90 treatment in experimental EBA supports the introduction of Hsp90 inhibitors, especially the nontoxic TCBL-145, into the clinical setting for the treatment of autoimmune disorders such as autoimmune blistering skin diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Rebecca Cames and Lidija Gutjahr for technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Excellence Cluster “Inflammation at Interfaces” (EXC 306/1), DFG LU 877/5-1, DFG SI 1281/1-1, DFG KA 3438/1-1, and Focus Program “Autoimmunity” at the University of Lübeck.
Authorship
Contribution: M.K. conceived the study, designed experiments, performed research, analyzed data, and wrote the manuscript; R. Manz designed, performed, and analyzed the experiments shown in Figure 5A-F; C.M.H. and J.W. designed, performed, and analyzed the experiments shown in Figure 5G-H; R. Müller, M.M., and E.S. designed, performed, and analyzed the experiments shown in Figure 6; C.S. produced TCBL-145; A.O., R.J.L., and D.Z. analyzed the research, performed statistical analyses, and contributed to composition of the paper. All authors critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Kasperkiewicz, Department of Dermatology, University of Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany; e-mail: Michael.Kasperkiewicz@uk-sh.de.
References
Author notes
R.J.L. and A.O. contributed equally to this study.