Abstract
Hematopoietic cell clusters associated with the midgestation mouse aorta, umbilical and vitelline arteries play a pivotal role in the formation of the adult blood system. Both genetic and live-imaging data indicate that definitive hematopoietic progenitor/stem cells (visualized as clusters) are generated from hemogenic endothelium. A 3-dimensional (3-D) whole embryo immunostaining and imaging technique has allowed quantitation and cartographic mapping of intravascular hematopoietic clusters. During this period the vitelline artery is most extensively remodeled, and several reports have suggested that vitelline remodeling leads to extravascular hematopoietic cluster emergence. Whether the earliest definitive progenitors/stem cells are intra or extra vascular could influence the process by which these cells migrate to the next hematopoietic territory, the fetal liver. Hence, by 3-D imaging we more closely examined hematopoietic clusters in the vitelline and associated connected small vessels and show here that hematopoietic clusters (particularly large clusters) are intravascular during the period of vascular remodeling.
Introduction
Development of the adult hematopoietic system occurs in close association with the vasculature of the mouse embryo.1-4 Following the first wave of primitive erythroid cell production, increasingly complex definitive hematopoietic progenitors are generated, with a final wave of emergence of hematopoietic stem cells (HSCs) that are the founders of the adult hematopoietic system.5-10 At the time of definitive hematopoietic progenitor and HSC generation, clusters of hematopoietic cells appear in the lumen of the dorsal aorta, and umbilical and vitelline arteries.11-13 These clusters are tightly associated with the vascular endothelium and arise from hemogenic endothelium lining the major embryonic vasculature.14
The Runx1 transcription factor, expressed in both hematopoietic cluster cells and endothelial cells,15,16 has been shown to be required in VE-cadherin expressing endothelial cells during midgestation for the generation of definitive hematopoietic stem/progenitor cells and for the formation of hematopoietic clusters.15,17,18 In addition, the Ly6A-GFP HSC marker, expressed by some cluster cells and hemogenic endothelial cells,1 has been used in recent live-imaging microscopy of the midgestation aorta to show the real-time transition of aortic hemogenic endothelial cells to hematopoietic cells within the lumen of this vessel.14 Other markers of emerging aortic hematopoietic cluster cells include c-Kit, CD41, CD45, and Flk1.13,14,16,19 Multicolour immunostaining analysis shows the heterogeneous cellular composition of intravascular clusters.13 A more broad view of clusters within the entire vasculature has been obtained with whole-mount 3-dimensional (3-D) confocal imaging mouse embryos between embryonic day (E)9.5-14.5.13 Cluster cartography and number quantifications show that hematopoietic clusters peak at E10.5 in the aorta, vitelline and umbilical arteries.13 The largest clusters are found in the vitelline and umbilical arteries, with up to 100 cells found in a vitelline artery cluster at E10.5.
During mouse midgestation the vitelline artery and many small vessels (localized between the aorta and the vitelline artery) undergo extensive vascular remodeling (E9-E11).11,13,20,21 Moreover, the midgut mesentery is growing within this region. Previously, blood islands have been described along the midgut mesentery and a recent cell tracing study suggests a nonluminal extravascular emergence of blood island/cluster cells.11,12,21 Since our multicolour 3-D imaging method detects not only hematopoietic clusters but also the complete vasculature,13 we performed extensive examinations of whole-mount immunostained embryos to determine the intra or extra vascular localization of the hematopoietic clusters in this region of remodeling. Our results show that Runx1 and c-Kit expressing hematopoietic clusters are intravascular.
Methods
Mice and embryos
Runx1-GFP transgenic mice carrying a Runx1 +24 mouse conserved noncoding element (mCNE)–enhanced green fluorescent protein (EGFP) reporter gene (C57BL/6 background) were described previously.22 Runx1 mutant mice (C57BL/6 background) have been described previously.23 Embryos were generated by timed matings between Runx1+/− males and females; C57BL/6 males and females; Runx1-GFP transgenic males and C57BL/6 females. Embryos were staged according to embryonic day, somite pairs (sp) and Thelier criteria (http://www.emouseatlas.org/emap/home.html). All animal experimentation was approved by the Ethical Board of the Experimental Animal Center at Erasmus MC.
Embryo staining and imaging
Whole-mount immunostaining and imaging were performed as described previously.13 In brief, immunostained embryos were mounted in a 1:2 mix of benzyl alcohol and benzyl benzoate (BABB) to increase the transparency of tissues, and were analyzed with a confocal microscope (Zeiss LSM 510 Meta, EC Plan-Neofluar 10×/numeric aperture [NA] 0.3, Plan-Neofluar 20×/NA 0.5). Pinhole diameter was set at 1 Airy unit and steps were 2.3-8.8 μm per z-section. Three-dimensional reconstructions were generated from z-stacks with LSM Image Browser (Zeiss).
Rat anti–mouse primary antibodies used were for c-Kit (2B8), CD34 (RAM34), and biotinylated anti-CD31(MEC 13.3; all from BD Biosciences). Secondary antibodies were goat anti–rat IgG-Alexa647 (Invitrogen) and Cy3-streptavidin (Jackson ImmunoResearch Laboratories) or goat anti–rat IgG-Alexa555 (Invitrogen). GFP was detected with rabbit anti-GFP antibody (MBL), followed by goat anti–rabbit IgG-Alexa647 (Invitrogen).
Results and discussion
Previously we developed a technique for imaging the complete vasculature of the midgestation mouse embryo by immunostaining with CD31 (marks all endothelial cells and hematopoietic clusters) and with c-Kit (marks hematopoietic clusters).13 We mapped the temporal and spatial distribution of intravascular clusters during the developmental times when hematopoietic cells emerge from hemogenic endothelium. Using this 3-D confocal imaging technique on immunostained, cleared (transparent) mouse embryos, we more closely examine whether hematopoietic clusters of the vitelline artery could be extravascular as proposed by others.11,12,21
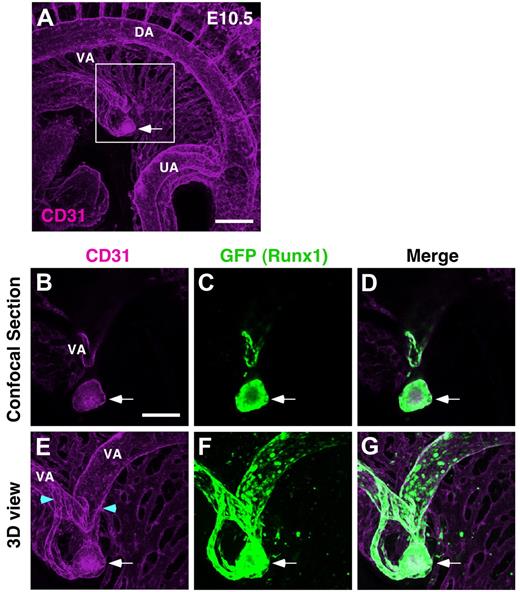
In E10 embryos, although the intensity of CD31 signal is weak in the c-Kit and CD31 double-staining combination (CD31-bio, streptavidin-Cy3), large clusters seem to be localized within small vessels around the vitelline artery (supplemental Figure 1, available on Blood Web site; see the Supplemental Materials link at the top of the online article). In parallel with c-Kit immunostaining, to more clearly visualize small blood vessels, Runx1-GFP transgenic embryos (marks hemogenic endothelial cells and hematopoietic clusters) were stained with CD31 and anti–rat IgG–Alexa555 (Figure 1 and supplemental Figure 2). In a representative confocal section showing the vitelline artery of an E10.5 Runx1 GFP:CD31 immunostained embryo, a large cluster appears to be extravascular (Figure 1B-D), and is in agreement with data of Zovein et al.21 However, when imaged in 3-D space, the large cluster is within a small vessel connected to the vitelline artery (Figure 1E-G and supplemental Videos 1-2). No extravascular clusters were detected around vitelline artery of any of the E10-10.5 embryos (n = 8) examined (Figure 1, supplemental Figure 2, and supplemental Table 1). To test the possibility that intravascular hematopoietic clusters from the vitelline artery may play a role in remodeling vasculature, we examined Runx1−/− embryos.13,15,18 The vascular plexus formation was normal in E10.5 Runx1−/− embryos in the absence of hematopoietic cluster generation (supplemental Figure 3).
Hematopoietic clusters within small vessels around vitelline artery at E10.5. Whole-mount immunostaining of E10.5 (35 sp) Runx1-GFP transgenic mouse for GFP and CD31 expression. (A) Three-dimensional imaging of CD31+ vasculature in E10.5 embryo. To clearly visualize the vascular plexus of mesentery, the z-stacks containing extra-embryonic part of vitelline artery and umbilical vein were removed from 3D projection. Scale bar: 200 μm. (B-G) Higher magnification views of boxed region in panel A. Confocal sections (B-D) and 3D reconstruction (E-G). Arrows indicate large hematopoietic cluster. Scale bar: 100 μm. Supplemental Videos 1 and 2 show a 3D image and sequential images along the z-axis, respectively. DA indicates dorsal aorta; VA, vitelline artery; and UA, umbilical artery. Blue arrowheads demarcate the area where small vessels emerge from VA.
Hematopoietic clusters within small vessels around vitelline artery at E10.5. Whole-mount immunostaining of E10.5 (35 sp) Runx1-GFP transgenic mouse for GFP and CD31 expression. (A) Three-dimensional imaging of CD31+ vasculature in E10.5 embryo. To clearly visualize the vascular plexus of mesentery, the z-stacks containing extra-embryonic part of vitelline artery and umbilical vein were removed from 3D projection. Scale bar: 200 μm. (B-G) Higher magnification views of boxed region in panel A. Confocal sections (B-D) and 3D reconstruction (E-G). Arrows indicate large hematopoietic cluster. Scale bar: 100 μm. Supplemental Videos 1 and 2 show a 3D image and sequential images along the z-axis, respectively. DA indicates dorsal aorta; VA, vitelline artery; and UA, umbilical artery. Blue arrowheads demarcate the area where small vessels emerge from VA.
These intra/extravascular data are interesting with respect to the ongoing hematopoietic colonization of the fetal liver during this period. As vitelline artery remodeling occurs in close proximity to the liver, interstitial migration of hematopoietic cells to the fetal liver has been proposed.21 A recent study showed the presence of large hematopoietic clusters on the cranial side (closest to the liver) of the vitelline artery at E11.21 When we examined E10.5 (n = 2), E11 (n = 2) and E11.5 (n = 5) embryos by 3-D imaging, we found no hematopoietic clusters localized on the cranial side of the vitelline artery (Figure 2). While it remains possible that the caudally localized intravascular hematopoietic clusters exit the vitelline artery by transendothelial migration (TEM; a process by which lymphocytes normally enter the interstitial tissue) and thereafter migration to the liver, this would most-likely involve only single cells and not large clusters, and additionally, these cells would extinguish Runx1 or c-Kit expression during the migration. Our data showing the intravascular localization of hematopoietic clusters favors the notion that hematopoietic progenitors/stem cells migrate though the circulation.
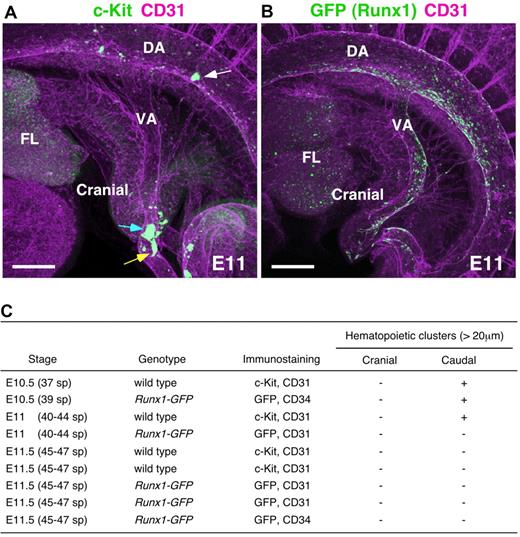
Hematopoietic clusters around vitelline artery at E11. (A,B) Whole-mount immunostaining of E11 (40-44 sp) embryos for (A) c-Kit and CD31 expression, and (B) Runx1-GFP and CD31 expression. White, blue, and yellow arrows indicate large hematopoietic clusters in the dorsal aorta, vitelline artery, and in the small vessel, respectively. Scale bars: 200 μm. (C) Large hematopoietic clusters (> 20 μm) observed in the cranial and caudal side of vitelline artery. DA indicates dorsal aorta; VA, vitelline artery; and FL, fetal liver.
Hematopoietic clusters around vitelline artery at E11. (A,B) Whole-mount immunostaining of E11 (40-44 sp) embryos for (A) c-Kit and CD31 expression, and (B) Runx1-GFP and CD31 expression. White, blue, and yellow arrows indicate large hematopoietic clusters in the dorsal aorta, vitelline artery, and in the small vessel, respectively. Scale bars: 200 μm. (C) Large hematopoietic clusters (> 20 μm) observed in the cranial and caudal side of vitelline artery. DA indicates dorsal aorta; VA, vitelline artery; and FL, fetal liver.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of the laboratory for comments and the National Institutes of Health (R37DK054077; E.D.), Netherlands BSIK Innovation Program 03040 (E.D.), and European Science Foundation Program EuroSTELLS 01-011(E.D.) for support.
National Institutes of Health
Authorship
Contribution: T.Y. designed and performed research, analyzed results and wrote the paper; C.E.L.N. and M.O. contributed analytical tools; and E.D. directed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elaine Dzierzak, Erasmus Medical Center, Erasmus MC Stem Cell Institute, Dept of Cell Biology, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: e.dzierzak@erasmusmc.nl.