In this issue of Blood, Hirai et al show that proteasome inhibitor bortezomib inhibits survival and function of plasmacytoid dendritic cells.1
Dendritic cells (DCs) are specialized antigen-presenting cells that play a central role in regulation of immunity.2 Their ability to carry out diverse roles is achieved in part via utilization of distinct subsets. Plasmacytoid DCs (pDCs) are a distinct subset that sense viruses and nucleic acid–containing complexes via intracellular Toll-like receptors (TLRs) to produce large quantities of type I interferons. Pathogen-derived or self nucleic acids meet TLRs in specialized vesicles called endolysosomes. To accomplish their function, TLRs must be properly transported from the endoplasmic reticulum (ER) to endolysosomes, an incompletely understood process that involves other cotransported proteins such as UNC32B.3 Coordination of this tango has implications for both viral immunity and autoimmunity.
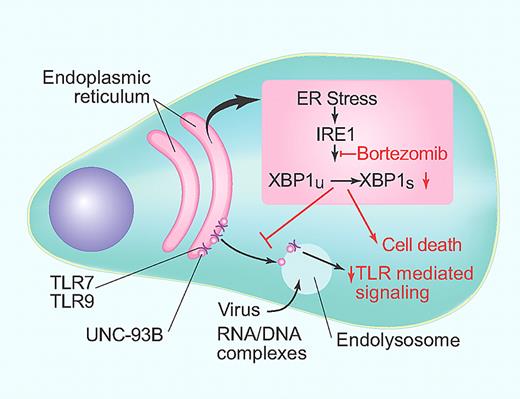
Proteasome inhibition in pDCs. ER stress leads to IRE1-mediated splicing of XBP1 as a part of unfolded protein response. Bortezomib-mediated inhibition of this pathway leads to induction of cell death and inhibits transport of Toll-like receptors to the endolysosome. (Professional illustration by Paulette Dennis.)
Proteasome inhibition in pDCs. ER stress leads to IRE1-mediated splicing of XBP1 as a part of unfolded protein response. Bortezomib-mediated inhibition of this pathway leads to induction of cell death and inhibits transport of Toll-like receptors to the endolysosome. (Professional illustration by Paulette Dennis.)
High level of protein turnover in DCs (including pDCs) creates a state of constant stress on the ER, which in turn initiates an adaptive response called the unfolded protein response (UPR).4 Activation of UPR involves an ER-resident kinase/endoribonuclease called IRE1. IRE1 leads to unconventional splicing of a transcription factor XBP1, leading to the formation of a “spliced form” XBP1s, critical for the activation of this arm of UPR.5 Prior studies have shown that this constitutive activation of UPR is important for survival of DCs, including pDCs in mice.4
Proteasome inhibitors such as bortezomib have emerged as effective therapies for plasma cell neoplasms.6 Besides DCs, plasma cells are the other major hematopoietic cell type that also depend on UPR for their survival.7 Prior studies have shown that bortezomib disrupts UPR in myeloma cells by inhibiting IRE1-mediated XBP1 splicing, leading to the death of tumor cells.7 As pDCs are also UPR dependent, it is not surprising that these cell types will also be similarly sensitive to bortezomib-induced cell death. Indeed, a prior study has shown that human pDCs are highly sensitive to bortezomib-induced cell death.8 Together with prior work, the data from Hirai et al show that bortezomib-induced suppression of XBP1 splicing leads to apoptosis of pDCs (see figure).1
The data from the present work also show that in addition to direct effect on survival, bortezomib also has a major effect on the capacity of pDCs to respond to TLR ligands. This is due to disruption with transport of TLRs to endolysosomes. These data therefore suggest that XBP1-induced chaperones may be important for transport of TLRs to endolysosomes. This is an elegant example of how pharmacologic inhibition of transport of a cellular sensor to the correct compartment may alter the capacity of the cell to respond. Inability to respond to TLR-driven signals may further impair the survival of pDCs in response to proteasome inhibition.
What are the clinical implications of this work? pDCs are thought to play an important role in defense against some viruses.9 pDCs are also emerging as critical players in the pathogenesis of several autoimmune states such as lupus and psoriasis, and the list is growing.9 For example, pDC-mediated release of type I interferon in response to nucleic acid-containing immune complexes is thought to be central to pathogenesis of lupus.9 Although bortezomib may be less amenable to long-term therapy, newer oral proteasome inhibitors may be suitable as novel therapeutics in interferon-driven or pDC-dependent autoimmunity. The effect of bortezomib on pDCs may also help understanding of the effects of this drug in the context of its current clinical use. For example, disabling pDC-mediated defenses may underlie bortezomib-induced susceptibility to viral infections observed in clinical studies.6 Interactions between tumor cells and pDCs may also be an important target of therapeutic effects of proteasome inhibitors in myeloma.8,10 The elegant biology of intracellular protein turnover and transport likely has many more surprises and therapeutic opportunities in store.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■