Abstract
Studies in animal models have shown that plasminogen activators bound to erythrocytes (RBC-PA) have an extended lifetime in the circulation and are safer than free PAs. RBC-PAs incorporate into nascent thrombi, which are focally lysed from within, an attractive thromboprophylactic option. In static systems, RBC-PAs cleave surrounding fibrin fibers, forming pores larger than the cells themselves, and move around the pore edges, enlarging them until eventual clot dissolution. We hypothesized that under flow in blood vessels, RBC-PAs form functional patent channels before clot dissolution. Here we used perfusion chambers to study clot lysis by RBC-PAs under static versus arterial and venous flow conditions. We found that flow decelerates bulk clot lysis but quickly generates patent channels filled with passing RBCs, via pore enlargement and merging in the direction of flow. Formation of such channels by RBC-PAs may help rescue ischemic tissue before bulk dissolution of potentially occlusive clots.
Introduction
Postsurgical patients at increased risk for both bleeding and thrombosis require safe short-term thromboprophylaxis.1 Anticoagulants and antiplatelet agents are only partially effective, pose a risk of bleeding (eg, heparin),2 or have a delayed onset (eg, warfarin). Thrombolytic therapy using plasminogen activation is confined to emergencies and is not used for short-term postsurgical thromboprophylaxis because of rapid drug clearance and unacceptable postoperative hemorrhage risk.1,3,4
Coupling to a red blood cell (RBC) converts tissue-type plasminogen activator and urokinase-type plasminogen activator from short-acting therapeutics to durable thromboprophylactic agents by prolonging circulation times and preventing penetration into tissues and existing hemostatic clots while delivering PA to the interior of nascent thrombi. Prophylactic administration of RBC-tissue-type plasminogen activator in animals restores blood flow within minutes after formation of occlusive arterial and venous thrombi5 and has been used for cerebrovascular thromboprophylaxis6 without increased propensity for bleeding.7 A single injection of a PA fused to an antibody fragment binding to mouse RBCs precluded the need for infusion of modified RBCs and conferred immediate and lasting thromboprophylaxis, thereby safely preventing arterial and venous occlusion in mouse models of vascular injury.8,9 Thus, short-term prophylaxis with RBC-PAs might prevent thrombotic occlusion while limiting the risk of bleeding.10
The mechanism by which RBC-bound PA mediates reperfusion is not intuitive because the enzyme is restricted to the cell surface. Previously, we used confocal microscopy of fluorescently labeled fibrin and RBCs to demonstrate that therapeutic lysis by soluble PA progressed gradually and uniformly, whereas prophylactic lysis by RBC-PAs embedded in clots occurred focally.11 Pores formed and enlarged within the fibrin network around RBC-PAs. The RBC-PAs then became highly mobile within these pores and moved quickly around the edges of dissolving fibers, propagating fibrinolysis by gradually enlarging the pores. This process continued until merging of the pores resulted in complete dissolution of the bulk clot.11
We posited that, in clots exposed to directional flow in blood vessels, these focal pores enlarge in the direction of flow and merge to form patent channels that permit blood components to traverse the clot before dissolution, thereby shortening ischemia. To test this hypothesis, we used confocal microscopy to monitor lysis and RBC passage through plasma clots containing RBC-PAs perfused with buffer at flow rates typical of venous and arterial vasculature.
Methods
All human and mouse blood samples were acquired and used according to accepted institutional review board and Institutional Animal Care and Use Committee protocols approved by review boards at the University of Pennsylvania. Clots were formed in Ibidi μ-slide VI0.1 flow chambers by mixing fluorescently labeled human platelet-poor plasma with Vybrant (R) DiD-labeled mouse RBCs carrying 70 000 molecules of urokinase-type plasminogen activator per RBC (RBC-PAs, 2% final hematocrit), 20mM CaCl2, and 0.1 U/mL human α-thrombin.8,11 Clot formation was complete 16 minutes after addition of thrombin. The clots were then perfused with Dulbecco phosphate-buffered saline containing 0.01% bystander RBCs (no PA attached) and 5% platelet-poor plasma at venous (160 s−1) or arterial (900 s−1) shear rates. Images were acquired by laser scanning and spinning disk confocal microscopy (image acquisition information in supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
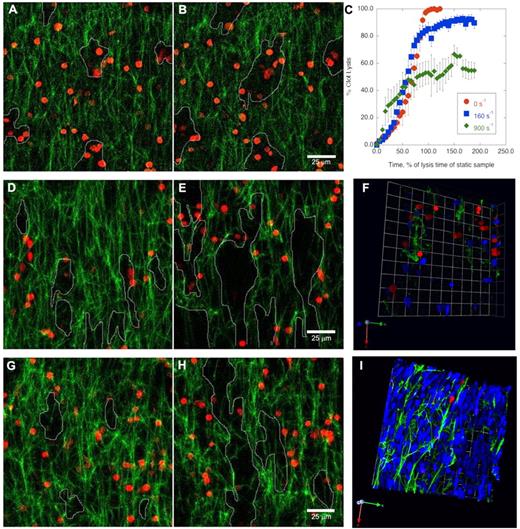
Perfusion of buffer through a clot with incorporated RBC-PAs changed the mechanism of clot dissolution. Lysis of a static clot by incorporated RBC-PAs proceeded as previously described11 with multilateral pore enlargement around RBC-PAs (Figure 1A-B; supplemental Movie 1). Bystander RBCs in the buffer placed at the clot edges did not enter the network during lysis. We plotted percent clot lysis, measured by the amount of fibrin network present at each time point, versus time (Figure 1C).
Shear stress changes the mechanism of prophylactic thrombolysis by incorporated RBC-PAs from multilateral lysis to formation of flow-directed patent channels. Z-projections of fibrin fiber (green) lysis by RBC-PAs (red) incorporated in clots before perfusion. (A-B) In the absence of flow, network pores (outlined) form multilaterally (panels A and B, 17.5 and 21 minutes, respectively). (C-I) Rate, extent, and mechanism of clot dissolution change with shear rate. (C) Percent clot lysis was determined by measuring the amount of network present at each time point and was plotted versus time. This plot combines results from 5 independent experiments, whereas the other panels in this figure provide representative examples. In the presence of venous shear (160 s−1), network pores merge in the direction of flow (from bottom to top; 17.5 and 21 minutes, panels D and E, respectively). (F) A 3-dimensional rendering of the network near the end of the venous shear experiment (35 minutes) shows the presence of patent channels (areas without green fibrin) and the passage of bystander RBCs (blue). Flow-directed channel formation becomes even more evident and faster in the presence of higher arterial shear (900 s−1, 17.5 and 21 minutes in panels G and H, respectively). (I) A 3-dimensional rendering shows that, under arterial shear, an increasing fraction of buffer-derived bystander RBCs traverse the clot via patent channels. Because so many bystander RBCs enter the clot at the higher shear rate, many of these RBCs get trapped inside the network pores (supplemental Data). Image acquisition information is available in supplemental Methods.
Shear stress changes the mechanism of prophylactic thrombolysis by incorporated RBC-PAs from multilateral lysis to formation of flow-directed patent channels. Z-projections of fibrin fiber (green) lysis by RBC-PAs (red) incorporated in clots before perfusion. (A-B) In the absence of flow, network pores (outlined) form multilaterally (panels A and B, 17.5 and 21 minutes, respectively). (C-I) Rate, extent, and mechanism of clot dissolution change with shear rate. (C) Percent clot lysis was determined by measuring the amount of network present at each time point and was plotted versus time. This plot combines results from 5 independent experiments, whereas the other panels in this figure provide representative examples. In the presence of venous shear (160 s−1), network pores merge in the direction of flow (from bottom to top; 17.5 and 21 minutes, panels D and E, respectively). (F) A 3-dimensional rendering of the network near the end of the venous shear experiment (35 minutes) shows the presence of patent channels (areas without green fibrin) and the passage of bystander RBCs (blue). Flow-directed channel formation becomes even more evident and faster in the presence of higher arterial shear (900 s−1, 17.5 and 21 minutes in panels G and H, respectively). (I) A 3-dimensional rendering shows that, under arterial shear, an increasing fraction of buffer-derived bystander RBCs traverse the clot via patent channels. Because so many bystander RBCs enter the clot at the higher shear rate, many of these RBCs get trapped inside the network pores (supplemental Data). Image acquisition information is available in supplemental Methods.
Exposing clots to 160 s−1 of shear (venous) led to enlargement over time of network pores that formed predominantly along the direction of flow (Figure 1D-E; supplemental Movie 2). Although individual fibrin fiber degradation occurred at a similar rate as in the absence of flow, not all fibers dissolved (Figure 1C). However, the holes that formed between the remaining fibrin strands merged into channels aligned along the direction of flow, through which bystander RBCs from the perfusion buffer passed unimpeded (Figure 1F). Although the perfusion buffer was supplemented with 5% platelet-poor plasma to provide limited plasminogen replenishment, the bulk of the original plasminogen and some RBC-PAs were eventually washed out, leading to an experimental artifact of lysis arrest over time.12 At arterial shear (900 s−1), patent channels formed even more rapidly and were quickly traversed by bystander RBCs (Figure 1G-I).
Therefore, within the short time interval tested (30-60 minutes), the final mass of fibrin lost under flow never exceeded 60% of the original mass. The initial clot dissolution rate was faster at higher shear but dropped off significantly once approximately 25% of the fibrin bulk was dissolved (Figure 1C). It was at this early time point when bystander RBCs began to perfuse the clot extensively (supplemental Movie 3).
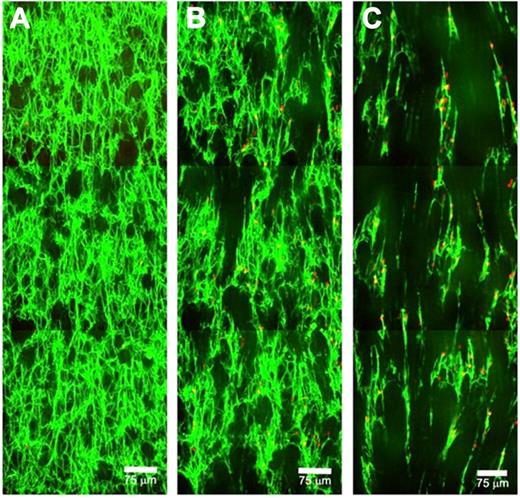
We then studied the fibrin network structural changes triggered by RBC-PAs using a piezo stage controller to image at 3 successive locations along the direction of flow (Figure 2; supplemental Movie 4). In accordance with the permeability data shown in Figure 1, channel formation under shear stress occurred before clot dissolution (Figure 2C). A few bystander RBCs were trapped in the residual fibrin, but most passed through the patent channels so rapidly they could not be captured in the Z-projections shown, although they are evident in individual slices. Pores enlarged and merged in the downstream direction (Figure 2B). As individual fibers were cut leaving one end free, these free ends oriented in the direction of flow, leading to alignment of the remaining fibrin mass. This response to lysis under flow triggered by incorporated RBC-PAs is a potential mechanism for the channel formation we observed. In our previous study, we observed pore enlargement triggered by RBC-PAs11 but without the directionality evident here.
Visualization of patent channels formed in clots by RBC-PAs under flow. Z-projections of 3 successive low-magnification images are stitched together with the direction of flow from bottom to top. (A) Just after the start of 160 s−1 shear (16 minutes), fibers and incorporated RBC-PAs are visible (both green), along with a few pores. (B) At 24 minutes, channels have begun to form from pores enlarging in the direction of flow. Some buffer-derived bystander RBCs (red) have entered clots and become trapped in the network. Fibers that have partially lysed tend to align in the direction of flow. (C) At 37 minutes, clearly visible patent channels have formed, and the strands of network that remain are aligned. Some RBC-PAs and buffer-derived RBCs are trapped in the remaining fibrin; the majority of the bystander RBCs traverse clots via channels, and many flow through the sample moving too fast to be captured in these Z-projections but can be detected in the videos (supplemental Data).
Visualization of patent channels formed in clots by RBC-PAs under flow. Z-projections of 3 successive low-magnification images are stitched together with the direction of flow from bottom to top. (A) Just after the start of 160 s−1 shear (16 minutes), fibers and incorporated RBC-PAs are visible (both green), along with a few pores. (B) At 24 minutes, channels have begun to form from pores enlarging in the direction of flow. Some buffer-derived bystander RBCs (red) have entered clots and become trapped in the network. Fibers that have partially lysed tend to align in the direction of flow. (C) At 37 minutes, clearly visible patent channels have formed, and the strands of network that remain are aligned. Some RBC-PAs and buffer-derived RBCs are trapped in the remaining fibrin; the majority of the bystander RBCs traverse clots via channels, and many flow through the sample moving too fast to be captured in these Z-projections but can be detected in the videos (supplemental Data).
These data indicate that a simple analysis of the loss of total fibrin network mass does not reveal some key, clinically relevant aspects of thrombolysis by RBC-PAs under flow. For example, although shear forces promote the formation of patent channels from initial holes surrounding individual PA-containing RBCs in all settings, the rate of complete clot dissolution by incorporated RBC-PA appears to be inversely related to shear (Figure 1C).
Under flow, rapid formation of channels in RBC-PA-containing clots allowed bystander RBCs to pass through a substantial mass of residual clot. Arterial shear provided faster channel formation versus venous shear, despite slower total clot dissolution. The channels observed resemble the fingerlike lysis patterns described by Blinc et al, who perfused free PAs through preformed whole blood clots,13,14 although there are differences between the experimental systems in PA size, stability, diffusion, impact on flow, and origin (effect of RBC-PAs originates from within the clot rather than the perfusion buffer). The current outcome correlates well with fast reperfusion of venous and arterial clots facilitated by RBC-PAs in vivo8 and indicates that RBC-PAs rapidly form patent channels in otherwise occlusive clots at both arterial and venous shear. This feature of thromboprophylaxis by RBC-PAs may further accelerate reperfusion and salvage of ischemic tissue even before clot dissolution is complete.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Samira Ismail and Dr Ronald Carnemolla for mouse blood.
This work was supported by the National Institutes of Health: HL30954 and HL090774 (J.W.W.), AHA SDG-0535258N (S.Z.), HL-076406, HL-077760, HD-057355, and HL-077760 (D.B.C.), and RO1-HL090697 (V.M.).
National Institutes of Health
Authorship
Contribution: K.C.G. performed and designed research, analyzed the results, and wrote the manuscript; and S.Z., D.B.C., V.M., and J.W.W. designed the research protocols, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Weisel, Department of Cell & Developmental Biology, University of Pennsylvania School of Medicine, 1054 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104-6058; e-mail: weisel@mail.med.upenn.edu.