Abstract
A highly complex network of coinhibitory and costimulatory receptors regulates the outcome of virus-specific CD8+ T-cell responses. Here, we report on the expression patterns of multiple inhibitory receptors on HIV-specific, cytomegalovirus-specific, and bulk CD8+ T-cell memory populations. In contrast to cytomegalovirus-specific CD8+ T cells, the majority of HIV-specific CD8+ T cells exhibited an immature phenotype and expressed Programmed Death-1, CD160 and 2B4 but not lymphocyte activation gene-3. Notably, before antiretroviral therapy, simultaneous expression of these negative regulators correlated strongly with both HIV load and impaired cytokine production. Suppression of HIV replication by antiretroviral therapy was associated with reduced surface expression of inhibitory molecules on HIV-specific CD8+ T cells. Furthermore, in vitro manipulation of Programmed Death-1 and 2B4 inhibitory pathways increased the proliferative capacity of HIV-specific CD8+ T cells. Thus, multiple coinhibitory receptors can affect the development of HIV-specific CD8+ T-cell responses and, by extension, represent potential targets for new immune-based interventions in HIV-infected persons.
Introduction
CD8+ T cells are a major component of the adaptive immune response against viruses and cancers. Although they form a heterogeneous population, they can be divided into distinct subsets that define the major steps in a process of memory T-cell differentiation.1,2 These multiple subsets display specific transcriptional programs and express distinct surface receptors and intracellular molecules, indicating quite different requirements for stimulation, survival, homing potential, and effector functions.3
In HIV infection, cellular immune responses fail to control the virus, and the majority of HIV-infected persons progress to develop AIDS.4 HIV-specific CD8+ T cells, which lack CD4+ T-cell help, express an exhausted phenotype characterized by an impaired ability to produce cytokines, and proliferate after in vitro stimulation.5 Furthermore, HIV-specific CD8+ T cells are sensitive to in vitro cell death,6 which further compounds their exhausted phenotype. Therefore, therapeutic interventions that target the survival and effector function of these cells could result in improved immune control of HIV infection.
Some of the mechanisms that lead to T-cell exhaustion7-9 are now being clarified. DNA microarray analyses of exhausted CD8+ T cells in murine models10 and humans11 suggest that T-cell exhaustion is the result of both active transcriptional suppression and defects in metabolism and cell signaling. Therefore, understanding how active inhibitory signals impact cellular immune responses may lead to the development of novel immunotherapeutic strategies.
An initial series of studies12-14 demonstrated that dysfunctional HIV-specific CD8+ T cells express high levels of Programmed Death-1 (PD-1), a major marker of virus-specific CD8+ T-cell exhaustion. Furthermore, a correlation between PD-1 expression on the surface of HIV-specific CD8+ T cells and either viral load or disease progression was observed.12,14 In addition, longitudinal analysis of HIV-infected subjects before and after the initiation of antiretroviral therapy (ART) showed that viral load reduction led to decreased levels of PD-1 expression on HIV-specific CD8+ T cells. Our group also demonstrated that PD-1–expressing CD8+ T cells are more susceptible to both spontaneous and Fas-mediated apoptosis.13 Cross-linking of PD-1 with an anti-PD-1 monoclonal antibody (mAb) preferentially triggered apoptosis in CD8+ T cells that expressed high levels of PD-1. Conversely, blockade of the PD-1 pathway with an anti-PD-L1 mAb enabled greater proliferation of HIV-specific CD8+ T cells.13
More recently, Blackburn et al reported that CD8+ T-cell responses during chronic viral infection in mice are regulated by complex patterns of coexpressed inhibitory receptors.15 In this latter study, several molecules that had previously been identified by DNA microarray analysis10 were found to be highly expressed on the surface of exhausted CD8+ T cells; these included PD-1, CD160,16,17 2B4,18 and lymphocyte activation gene-3 (LAG-3).19,20 Furthermore, it appears that the greater the coexpression of these inhibitory receptors, the greater the degree of exhaustion exhibited by virus-specific CD8+ T cells in both mice and humans.21,22
In this study, we examined the simultaneous expression patterns of PD-1, CD160, 2B4, and LAG-3 on CD8+ T-cell populations with defined virus-derived antigen specificities. The expression of inhibitory receptors varied with antigen specificity and T-cell differentiation status in HIV-infected persons. Moreover, the simultaneous expression of these molecules correlated directly with HIV load and inversely with the multiplicity of functional outputs exhibited by HIV-specific CD8+ T cells reexposed to cognate antigen. In addition, the proliferative capacity of HIV-specific CD8+ T cells was restored by blocking both PD-1/PD-L1 and 2B4/CD48 interactions.
Methods
Study subjects and cell culture
HIV-1–infected antiretroviral-naive male and nonpregnant female subjects with a minimum age of 13 years and plasma HIV RNA levels more than 2000 copies/mL were enrolled.23 The study protocol was approved by an Institutional Review Board or ethics committee at each participating site. All participants provided written informed consent, in accordance with the Declaration of Helsinki. AIDS Clinical Trial Group A5142 was a phase 3, randomized, multicenter, open-label trial.24 Longitudinal cryopreserved peripheral blood mononuclear cell (PBMC) samples were collected from HIV+ (n = 85) and HIV− (n = 19) persons. All available subjects from AIDS Clinical Trial Group A5142 who had adequate stored samples from baseline and week 48 with more than 85% viability and HIV pVL levels less than 50 copies after week 24 were studied. The median pre-ART CD4+ T-cell count of the 85 HIV+ persons was 227.5 cells/μL (range, 6-1052 cells/μL), and the median pre-ART pVL was 38 905 RNA copies/mL plasma (range, 1567-1 412 538 RNA copies/mL plasma). An additional 11 HIV+ persons were recruited from Drexel University College of Medicine, Philadelphia, PA; freshly isolated PBMCs from these persons were used for functional analysis of inhibitory receptor blockade. A further 11 HIV+ persons (3 viremic and 8 aviremic) were recruited from Duke University Medical Center (Durham, NC); PBMCs from these persons were included in the combined intracellular cytokine and tetramer staining experiments. PBMCs were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen).
Antibodies
The following directly conjugated mAbs were used: (1) CD3-Cy7allophycocyanin (APC), IL2-Cy5.5peridinin chlorophyll protein, interferon-γ (IFN-γ)–APC and tumor necrosis factor-α (TNF-α)–Cy7PE (BD Biosciences); (2) CD45RO-TexasRedPE, 2B4-Cy5PE (clone C1.7), CD160-PE (clone BY55), and CD27-Alexa700APC (Beckman Coulter); (3) CD4-Qdot605 (Invitrogen); and (4) LAG-3-fluorescein isothiocyanate (FITC; clone 17B4) and IFN-γ–Pacific Blue (eBioscience). The following mAbs were conjugated in our laboratory: CCR7-Pacific Blue, CD14-Pacific Blue, CD19-Pacific Blue, CD8-Qdot585, and CD8-Qdot705. Unconjugated mAbs were obtained from BD Biosciences. Pacific Blue was obtained from Invitrogen. Quantum dots were obtained from Invitrogen. The violet (ViViD), Aqua amine reactive viability dyes were obtained from Invitrogen. Biotinylated PD-1 mAb (clone; BAF 1086) was obtained from R&D Systems, and conjugated streptavidin (Cy7PE or Qdot655) was obtained from Invitrogen.
Polychromatic flow cytometry
Cells were analyzed with a modified LSRII flow cytometer (BD Immunocytometry Systems) as described previously.25 Briefly, 3 × 106 PBMCs were incubated in 1 mL of medium containing monensin (0.7 μg/mL; BD Biosciences) and brefeldin A (10 μg/mL; Sigma-Aldrich) in the absence or presence of peptides (15mers overlapping by 11 residues) corresponding to full-length HIV-1 Gag or cytomegalovirus (CMV) pp65 (2 μg/mL each peptide, 5 μL/mL; National Institutes of Health AIDS Research and Reference Reagent Program) for 6 hours. After washing, cells were surface stained for PD-1, CD160, 2B4, LAG-3, CD4, CD8, CD27, CD45RO, CCR7, and CD14/CD19; ViViD or Aqua was used to exclude dead cells from the analysis. After permeabilization (Cytofix/Cytoperm kit; BD Biosciences), cells were stained for CD3, IFN-γ, IL-2, and TNF-α. Between 300 000 and 2 × 106 events were collected in each case. Electronic compensation was conducted with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. Data were analyzed using FlowJo Version 9.0.1 (TreeStar). Forward scatter area versus forward scatter height was used to gate out cell aggregates. CD14+, CD19+, and dead cells were removed from the analysis to reduce background staining. The combinations ViViD/CD14–Pacific Blue/CD19–Pacific Blue/CD3-Cy7APC/CD4-Qdot605/CD8-Qdot705/CD27-Alexa700APC/CD45RO-TexasRedPE/PD-1-SA-Qdot655/2B4-Cy5PE/CD160-PE/LAG-3-FITC, Aqua/CD3-Cy7APC/CD4-Qdot605/CD8-Qdot585/CD27-Alexa700APC/CD45RO-TexasRedPE/CCR7–Pacific Blue/PD-1-SA-Qdot655/2B4-Cy5PE/CD160-PE/LAG-3-FITC/TNF-α–Cy7PE/IL2-Cy5.5peridinin chlorophyll protein/IFN-γ–APC, and Aqua/CD3-Cy7APC/CD4-Qdot605/CD8-Qdot585/CD27-Alexa700APC/CD45RO-TexasRedPE/PD-1-SA-Qdot655/2B4-Cy5PE/CD160-PE/LAG-3-FITC/TNF-α–Cy7PE/IL2-Cy5.5peridinin chlorophyll protein/IFN-γ–Pacific Blue/tetramer-APC were used for further characterization.
Peptide-MHC class I tetramers
Tetrameric peptide/human leukocyte antigen (HLA) class I tetramers conjugated to APC were produced as described previously.26 The following complexes were used in this study: HIV-1 Gag SLYNTVATL(SL9)/HLA A*0201, CMV pp65 NLVPMVATV(NV9)/HLA A*0201, HIV-1 Nef TPQVPLRPM(TM9)/HLA B*0702, CMV pp65 TPRVTGGGAM(TM10)/HLA B*0702, and HIV-1 Gag TSTLQEQIAW(TW10)/HLA B*5701.
Proliferation studies
Carboxyfluorescein succinimidyl ester dilution assays to examine CD8+ T-cell proliferative responses were performed as described previously, using a final concentration of 0.2 μg/mL peptide with the costimulatory mAbs CD28 and CD49d at 1 μg/mL each (BD Biosciences).25 Briefly, freshly isolated PBMCs were incubated with or without the HIV-1 Gag peptide pool containing isotype control antibody (IgG2b clone MPC.11; 10 μg/mL), purified anti–PD-L1 (10 μg/mL; eBioscience), purified anti-CD48 (5 μg/mL; eBioscience), or purified anti–PD-L1 plus anti-CD48. Cells were incubated for 6 days, stained with surface antibodies, and the percentage of carboxyfluorescein succinimidyl esterlow CD8+ T cells was analyzed by flow cytometry. For some experiments, the SL9, a gag-epitope peptide, was used for stimulation. The production of IFN-γ and TNF-α was also analyzed after 6 days of culture in some experiments. In this case, cells were restimulated on day 6 with the same peptide pool and cytokine production was detected by intracellular staining.
Statistical analysis
Experimental variables were analyzed using the nonparametric Mann-Whitney U test, the Wilcoxon matched-pairs signed rank test, and the Friedman test; correlations were performed using the nonparameric Spearman rank test. Bars represent median values. P values < .05 were considered significant. The GraphPad Prism statistical analysis program (Version 5.0c; GraphPad Software) was used throughout. Analysis and graphical representation of cytokine production in relation to PD-1, CD160, 2B4, and LAG-3 expression were conducted using the data analysis program Simplified Presentation of Incredibly Complex Evaluations (Version 5.05013) provided by M. Roederer (National Institutes of Health, Bethesda, MD).
Results
Expression patterns of inhibitory receptors vary across distinct memory CD8+ T-cell subsets
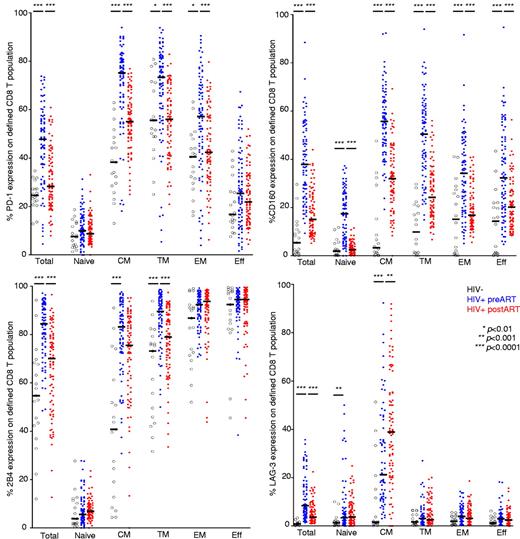
To characterize the role of inhibitory receptors in HIV+ persons, we first analyzed the expression patterns of several costimulatory and coinhibitory receptors on virus-specific CD8 T cells. Our preliminary studies showed negligible expression of Tim-3 and CTLA-4 on these cells (both surface and intracellular; data not shown). We therefore focused our analyses in the expression of PD-1, CD160, 2B4, and LAG-3 on total and virus-specific CD8+ T cells from infected donors before and after ART treatment (AIDS Clinical Trial Group A5142 cohort).23,24 Representative gating schemes for the flow cytometric analysis of individual surface molecule expression with respect to CD8+ T-cell maturation status are shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The surface markers CD27, CD45RO, and CCR7 were used to define memory populations. Naive CD8+ T cells, the population with the lowest levels of inhibitory receptors, were used as a reference to define cells with high expression levels of the individual molecules tested. As described previously, we found that PD-1 was expressed primarily on central memory (CM), transitional memory (TM), and effector memory (EM) CD8+ T cells27 ; CD160 was expressed mainly on CM and TM CD8+ T cells (Figure 1). In contrast, the expression of 2B4 on CD8+ T cells increased progressively with maturation status; thus, the EM and effector (Eff) populations expressed the highest levels of 2B4. Although the majority of CD8+ T cells stimulated in vitro expressed high levels of LAG-3 (supplemental Figure 2), ex vivo measurements of this molecule revealed elevated expression only within the CM population.
Expression patterns of inhibitory receptors vary across distinct memory CD8+ T-cell subsets. Pooled data showing the percentage of CD8+ T cells expressing PD-1, CD160, 2B4, and LAG-3 within the total CD8+ T-cell pool from healthy donors (n = 19) and HIV+ persons before and after ART (n = 85). PD-1+, CD160+, 2B4+, and LAG-3+ expression in naive and memory CD8+ T-cell subsets from healthy donors and HIV+ persons before and after ART is also shown. Horizontal lines indicate median values. The P values were calculated using a combination of a paired test for the pre- and post-initiation of therapy measurements, and Mann-Whitney unpaired test to compare the HIV-negative group with HIV+ pre-ART of measures. *P < .01, **P < .001, ***P < .0001.
Expression patterns of inhibitory receptors vary across distinct memory CD8+ T-cell subsets. Pooled data showing the percentage of CD8+ T cells expressing PD-1, CD160, 2B4, and LAG-3 within the total CD8+ T-cell pool from healthy donors (n = 19) and HIV+ persons before and after ART (n = 85). PD-1+, CD160+, 2B4+, and LAG-3+ expression in naive and memory CD8+ T-cell subsets from healthy donors and HIV+ persons before and after ART is also shown. Horizontal lines indicate median values. The P values were calculated using a combination of a paired test for the pre- and post-initiation of therapy measurements, and Mann-Whitney unpaired test to compare the HIV-negative group with HIV+ pre-ART of measures. *P < .01, **P < .001, ***P < .0001.
ART decreases inhibitory receptor expression on memory CD8+ T cells in HIV+ persons
In this cohort, plasma viral load (pVL) was consistently less than 50 copies/mL in all persons between week 24 and week 48 after the initiation of ART. Reduced expression of all inhibitory molecules (PD-1, CD160, 2B4, and LAG-3) within the total CD8+ T-cell pool was observed in HIV+ persons on ART during this period compared with baseline levels before treatment (P < .0001 in each case for PD-1, CD160, 2B4, and LAG-3; Figure 1). Of note, although ART reduced the elevated expression of CD160 on all CD8+ T cells, this decrease was especially marked on naive CD8+ T cells; thus, CD160 levels decreased to parallel those observed on naive CD8+ T cells from HIV− persons, suggesting that CD160 may be an antigen-independent marker of general activation. Expression levels of PD-1 on CD8+ T cells on CM, TM, and EM compartments were reduced on ART, as was 2B4 expression on TM cells. Paradoxically, LAG-3 expression on CD8+ T cells with a CM phenotype increased on ART.
HIV-specific CD8+ T cells express increased levels of inhibitory receptors in the absence of ART
Next, we investigated the expression levels of all 4 inhibitory molecules on CD8+ T cells specific for HIV and CMV. Virus-specific CD8+ T cells were detected by intracellular staining for IFN-γ in PBMC preparations stimulated with overlapping peptide pools spanning the HIV-1 Gag and CMV pp65 proteins (supplemental Figure 3). The expression profile of these inhibitory molecules on HIV-specific CD8+ T cells was similar regardless of the peptide pool (Gag, Pol, Env, or Nef) used for stimulation (data not shown). On this basis, the Gag peptide pool was selected for the detection of HIV-specific CD8+ T cells.
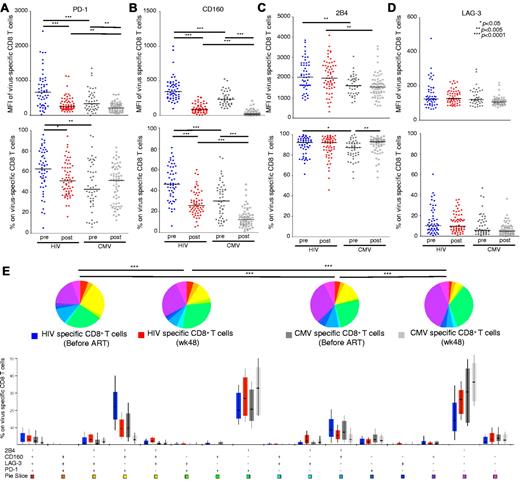
Analysis of pre-ART samples showed that HIV-specific CD8+ T cells expressed significantly higher levels of PD-1 compared with CMV-specific CD8+ T cells (mean fluorescence intensity [MFI], P < .0001; percentage, P = .0014; Figure 2A), in line with previously reported data.13 After ART, PD-1 expression on HIV-specific CD8+ T cells decreased (MFI, P < .0001; percentage, P = .0077). Although the MFI of PD-1 on CMV-specific CD8+ T cells before ART was higher compared with that in post-ART samples (P = .0017), the percentage of PD-1+ CMV-specific CD8+ T cells did not change significantly (P = .8142). CD160 expression was substantially higher on HIV-specific compared with CMV-specific CD8+ T cells in pre-ART samples (MFI, P < .0001; percentage, P < .0001; Figure 2B). ART reduced the expression of CD160 on both HIV-specific and CMV-specific CD8+ T cells (P < .0001 for all comparisons; Figure 2B). The expression intensity (MFI) of 2B4 was also consistently higher on HIV-specific compared CMV-specific CD8+ T cells (P = .0016 for pre-ART comparison; P = .0002 for post-ART comparison; Figure 2C). However, the percentage of 2B4+ cells was similar in the HIV-specific and CMV-specific CD8+ T-cell populations (P = .0247 for pre-ART comparison; P = .5490 for post-ART comparison). No differences between HIV-specific and CMV-specific CD8+ T cells were observed with respect to LAG-3 expression, either in pre- or post-ART samples (Figure 2D).
HIV-specific CD8+ T cells express high levels of inhibitory molecules before ART. (A-D) Comparisons of MFI and percentage of PD-1, CD160, 2B4, and LAG-3 expression on HIV-specific and CMV-specific CD8+ T cells in matched samples from 85 HIV+ persons. HIV-specific (n = 52, blue circles) and CMV-specific (n = 60, dark gray circles) CD8+ T cells before ART; HIV-specific (n = 59, red circles) and CMV-specific (n = 62, light gray circles) CD8+ T cells after ART. Each dot represents one person at one time point. (E) Percentage expression of inhibitory molecules on HIV-specific CD8+ T cells before ART (n = 52, blue bars) and after ART (n = 59, red bars), and on CMV-specific CD8+ T cells before ART (n = 60, dark gray bars) and after ART (n = 62, light gray bars) are shown for each of the 16 phenotypic subsets based on the expression profiles of PD-1, CD160, 2B4, and LAG-3. *P < .01, **P < .001, ***P < .0001.
HIV-specific CD8+ T cells express high levels of inhibitory molecules before ART. (A-D) Comparisons of MFI and percentage of PD-1, CD160, 2B4, and LAG-3 expression on HIV-specific and CMV-specific CD8+ T cells in matched samples from 85 HIV+ persons. HIV-specific (n = 52, blue circles) and CMV-specific (n = 60, dark gray circles) CD8+ T cells before ART; HIV-specific (n = 59, red circles) and CMV-specific (n = 62, light gray circles) CD8+ T cells after ART. Each dot represents one person at one time point. (E) Percentage expression of inhibitory molecules on HIV-specific CD8+ T cells before ART (n = 52, blue bars) and after ART (n = 59, red bars), and on CMV-specific CD8+ T cells before ART (n = 60, dark gray bars) and after ART (n = 62, light gray bars) are shown for each of the 16 phenotypic subsets based on the expression profiles of PD-1, CD160, 2B4, and LAG-3. *P < .01, **P < .001, ***P < .0001.
HIV-specific and CMV-specific CD8+ T cells display distinct patterns of inhibitory receptor expression
In further experiments, we determined the simultaneous expression of PD-1, CD160, 2B4, and LAG-3 on HIV-specific and CMV-specific CD8+ T cells (Figure 2E). Of the 16 potential permutational expression patterns of these 4 inhibitory molecules, 3 predominated. We therefore evaluated virus-specific CD8+ T cells that fell within these 3 major populations: PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3−. The percentage of HIV-specific CD8+ T cells that expressed a PD-1+CD160+2B4+LAG-3− phenotype was significantly higher compared with CMV-specific CD8+ T cells (P < .0001). After ART, the percentage of both HIV-specific and CMV-specific CD8+ T cells bearing a PD-1+CD160+2B4+LAG-3− expression pattern decreased dramatically. The majority of CMV-specific CD8+ T cells were PD-1−CD160−2B4+LAG-3−; this phenotypic pattern increased within both the HIV-specific and CMV-specific CD8+ T-cell populations after ART (Figure 2E).
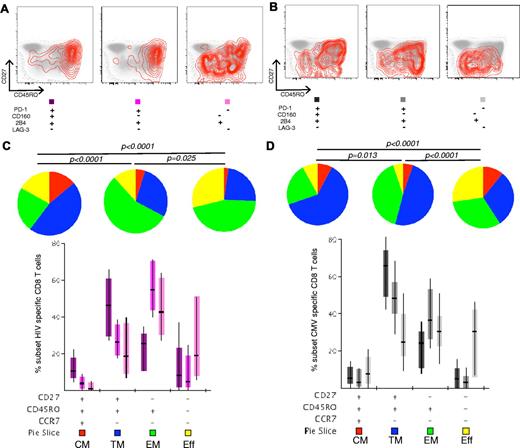
Next, we assessed the maturation level of CD8+ T cells with PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression patterns (Figure 3A-B). The PD-1+CD160+2B4+LAG-3− expression pattern was associated with a relatively immature differentiation status (CCR7lowCD27hiCD45ROhi); the majority of HIV-specific CD8+ T cells accumulate in this phenotypic compartment.28,29 Single expression of 2B4, however, characterized a highly differentiated population (CCR7lowCD27lowCD45ROlow) both in HIV-specific and CMV-specific CD8+ T-cell populations (P < .0001 in each case; Figure 3C-D). Thus, our data suggest a relationship between the expression of inhibitory receptors and the level of maturation. However, it is not clear whether these molecules play an active role in regulating the differentiation process of virus-specific CD8+ T cells. Next, the association between the simultaneous expression of PD-1, CD160, and 2B4 and activation level of HIV-specific CD8+ T cells was investigated. A significant correlation was found between this “coinhibitory pattern” and the expression of CD38 (P = .03). No correlation was found between this pattern and HLADR or CD57, a marker of senescence.
Virus-specific CD8+ T cells become less exhausted with increasing maturity. (A-B) The surface phenotype of HIV-specific and CMV-specific CD8+ T cells is shown for representative exhausted populations (PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3−) from a single HIV+ person by overlaying responding cells (red contour plots) on density plots of the corresponding total CD8+ T-cell populations for CD27 and CD45RO expression. (C-D) Maturation decreases inhibitory molecule expression on PD-1+CD160+2B4+LAG-3− (dark purple), PD-1+CD160−2B4+LAG-3− (purple), and PD-1−CD160−2B4+LAG-3− (light purple) HIV-specific CD8+ T cells, and PD-1+CD160+2B4+LAG-3− (dark gray), PD-1+CD160−2B4+LAG-3− (gray), and PD-1−CD160−2B4+LAG-3− (light gray) CMV-specific CD8+ T cells, in HIV+ persons before ART (n = 47 for HIV-specific CD8+ T cells; n = 60 for CMV-specific CD8+ T cells). The phenotypic complexity of virus-specific CD8+ T cells was assessed by analyzing the individual phenotypic patterns. The y-axis displays the percentage of each phenotypic pattern, the composition of which is denoted on the x-axis with + for the presence of CD27, CD45RO, and CCR7 on PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− virus-specific CD8+ T cells. The proportion of total memory CD8+ T cells accounted for by each phenotypic pattern, together with the median and interquartile ranges, are shown. The phenotypic patterns are grouped and color-coded according to phenotype and summarized in pie chart form; each pie slice represents the mean proportion of total memory CD8+ T cells for each inhibitory receptor expression pattern. Red represents CM; blue, TM; green, EM; and yellow, Eff.
Virus-specific CD8+ T cells become less exhausted with increasing maturity. (A-B) The surface phenotype of HIV-specific and CMV-specific CD8+ T cells is shown for representative exhausted populations (PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3−) from a single HIV+ person by overlaying responding cells (red contour plots) on density plots of the corresponding total CD8+ T-cell populations for CD27 and CD45RO expression. (C-D) Maturation decreases inhibitory molecule expression on PD-1+CD160+2B4+LAG-3− (dark purple), PD-1+CD160−2B4+LAG-3− (purple), and PD-1−CD160−2B4+LAG-3− (light purple) HIV-specific CD8+ T cells, and PD-1+CD160+2B4+LAG-3− (dark gray), PD-1+CD160−2B4+LAG-3− (gray), and PD-1−CD160−2B4+LAG-3− (light gray) CMV-specific CD8+ T cells, in HIV+ persons before ART (n = 47 for HIV-specific CD8+ T cells; n = 60 for CMV-specific CD8+ T cells). The phenotypic complexity of virus-specific CD8+ T cells was assessed by analyzing the individual phenotypic patterns. The y-axis displays the percentage of each phenotypic pattern, the composition of which is denoted on the x-axis with + for the presence of CD27, CD45RO, and CCR7 on PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− virus-specific CD8+ T cells. The proportion of total memory CD8+ T cells accounted for by each phenotypic pattern, together with the median and interquartile ranges, are shown. The phenotypic patterns are grouped and color-coded according to phenotype and summarized in pie chart form; each pie slice represents the mean proportion of total memory CD8+ T cells for each inhibitory receptor expression pattern. Red represents CM; blue, TM; green, EM; and yellow, Eff.
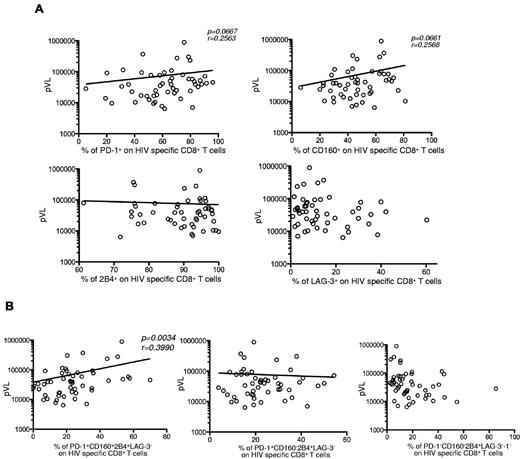
The frequency of hyperexhausted HIV-specific CD8+ T cells correlates with viremia
Previously published data have shown a correlation between pVL and PD-1 expression on HIV-specific CD8+ T cells.12,14 Therefore, we analyzed the relationship between pVL and the expression of inhibitory molecules in HIV+ persons before ART. No significant correlation was detected between pVL and single expression of any of the 4 inhibitory receptors on HIV-specific CD8+ T cells (Figure 4A). However, a significant correlation was found between pVL and the simultaneous expression of PD-1, CD160, and 2B4 on HIV-specific CD8+ T cells (P = .0034). No correlation was observed for the other 2 major populations, PD-1+CD160−2B4+LAG-3− and PD-1−CD160−2B4+LAG-3− (Figure 4B). These data imply that the simultaneous expression of PD-1, CD160, and 2B4 may better characterize the exhausted phenotype of HIV-specific CD8+ T cells under conditions of chronic virus-specific stimulation.
Hyperexhausted HIV-specific CD8+ T cells correlate with viremia. (A) Percentage of single inhibitory molecule expression on HIV-specific CD8+ T cells as a function of pVL (n = 52). (B) Percentage of PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression profiles on HIV-specific CD8+ T cells as a function of pVL (n = 47). Lines indicate correlations detected by Prism software Version 5.0c.
Hyperexhausted HIV-specific CD8+ T cells correlate with viremia. (A) Percentage of single inhibitory molecule expression on HIV-specific CD8+ T cells as a function of pVL (n = 52). (B) Percentage of PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression profiles on HIV-specific CD8+ T cells as a function of pVL (n = 47). Lines indicate correlations detected by Prism software Version 5.0c.
Simultaneous expression of inhibitory receptors correlates inversely with polyfunctionality
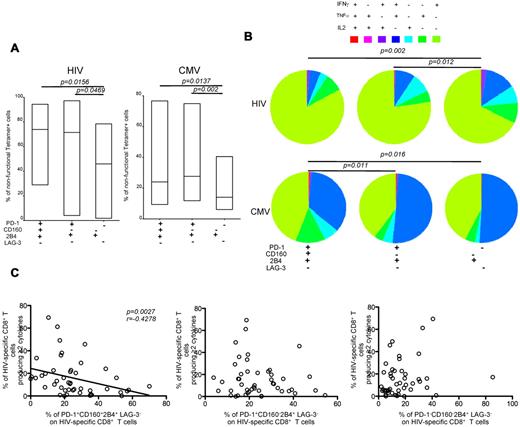
Next, we investigated the capacity of virus-specific CD8+ T cells to produce cytokines with respect to the expression of inhibitory molecules. Cells were stimulated with cognate peptides, and total antigen-specific CD8+ T-cell populations (tetramer+cytokine+, tetramer+cytokine−, and tetramer−cytokine+) were analyzed for inhibitory molecule expression patterns (Figure 5A). The frequencies of nonfunctional HIV-specific CD8+ T cells within the PD-1+CD160+2B4+LAG-3− and PD-1+CD160−2B4+LAG-3− compartments were significantly higher compared with the corresponding frequencies in the PD-1−CD160−2B4+LAG-3− compartment (P = .0156 and P = .0469, respectively; Figure 5A). The frequencies of nonfunctional CMV-specific CD8+ T cells within the PD-1+CD160+2B4+LAG-3− and PD-1+CD160−2B4+LAG-3− compartments were also higher compared with the corresponding frequencies in the PD-1−CD160−2B4+LAG-3− compartment (P = .0137 and P = .002, respectively; Figure 5A).
Inhibitory marker coexpression correlates inversely with polyfunctionality. (A) 10 HIV+ subjects from Duke University were studied. Representative plots of intracellular cytokine staining combined with SL9/HLA A*0201, NV9/HLA A*0201, TM9/HLA B*0702, TM10/HLA B*0702, or TW10/HLA B*5701 tetramer staining of CD8+ T cells are shown. Cells were independently stimulated with individual epitopic peptides for 6 hours. Pooled data lower panels represent the percentage of nonfunctional CD8+ T cells with PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression profiles within tetramer+ or any cytokine+ populations (HIV-specific responses, n = 7; CMV-specific responses, n = 10). (B) The fraction of the total response composing cells expressing each of the 7 possible combinations of IFN-γ, IL-2, and TNF-α production within the PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− populations. Cells were stimulated with the HIV-1 Gag peptide pool (n = 47) or the CMV pp65 peptide pool (n = 60), respectively. (C) Comparisons between polyfunctionality and the percentage of PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression profiles on HIV-specific CD8+ T cells as a function of pVL (n = 47). Lines indicate correlations detected by Prism software Version 5.0c.
Inhibitory marker coexpression correlates inversely with polyfunctionality. (A) 10 HIV+ subjects from Duke University were studied. Representative plots of intracellular cytokine staining combined with SL9/HLA A*0201, NV9/HLA A*0201, TM9/HLA B*0702, TM10/HLA B*0702, or TW10/HLA B*5701 tetramer staining of CD8+ T cells are shown. Cells were independently stimulated with individual epitopic peptides for 6 hours. Pooled data lower panels represent the percentage of nonfunctional CD8+ T cells with PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression profiles within tetramer+ or any cytokine+ populations (HIV-specific responses, n = 7; CMV-specific responses, n = 10). (B) The fraction of the total response composing cells expressing each of the 7 possible combinations of IFN-γ, IL-2, and TNF-α production within the PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− populations. Cells were stimulated with the HIV-1 Gag peptide pool (n = 47) or the CMV pp65 peptide pool (n = 60), respectively. (C) Comparisons between polyfunctionality and the percentage of PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− expression profiles on HIV-specific CD8+ T cells as a function of pVL (n = 47). Lines indicate correlations detected by Prism software Version 5.0c.
Previous studies30 have revealed the importance of T-cell polyfunctionality, defined by the multiplicity of antigen-specific cytokine production and other effector functions, in HIV pathogenesis. Such functional profiling has been used as an indicator of T-cell quality, and an inverse correlation has been found between pVL and the polyfunctionality of HIV-specific CD8+ T cells in HIV-infected persons.5 Therefore, we analyzed the polyfunctionality of HIV-specific and CMV-specific CD8+ T cells with PD-1+CD160+2B4+LAG-3−, PD-1+CD160−2B4+LAG-3−, and PD-1−CD160−2B4+LAG-3− patterns of inhibitory receptor expression (Figure 5B). HIV-specific CD8+ T cells with a PD-1+CD160+2B4+LAG-3− phenotype were less polyfunctional compared with HIV-specific CD8+ T cells with a PD-1−CD160−2B4+LAG-3− phenotype (n = 47, P = .002). Similar findings applied to CMV-specific CD8+ T cells (n = 60, P = .016). Moreover, there was a significant inverse correlation between the percentage of PD-1+CD160+2B4+LAG-3− cells within the HIV-specific CD8+ T-cell population and the percentage of polyfunctional HIV-specific CD8+ T cells (P = .0027; Figure 5C). This was not the case, however, for the PD-1+CD160−2B4+LAG-3− and PD-1−CD160−2B4+LAG-3− HIV-specific CD8+ T-cell populations (Figure 5C). Thus, the simultaneous expression of several inhibitory molecules correlates inversely with the capacity of CD8+ T cells to produce cytokines.
Blockade of both PD-1 and 2B4 engagement restores HIV-specific CD8+ T-cell proliferation
The proliferative potential of AIDS virus-specific CD8+ T cells can be restored by manipulating the interaction between PD-1 and its ligand, PD-L1, both in vitro12-14 and in vivo.31 To extend these observations, we investigated the effects of additional inhibitory pathway manipulations on the proliferation profile of virus-specific CD8+ T cells in vitro (Figure 6). Our preliminary studies showed a similar positive effect of blocking PD-1/PD-L1 in proliferation of virus-specific CD8 T cells in the absence or presence of anti-CD28/49d costimulation. The addition of anti-CD28/49d, however, resulted in higher proliferation rates (data not shown). Simultaneous blockade of the PD-1/PD-L1 and 2B4/CD48 interactions exhibited a superior effect in terms of restoring the proliferation of HIV-specific CD8+ T cells compared with PD-1/PD-L1 blockade alone (n = 11, P < .05; Figure 6B) when cells were stimulated with viral peptide pools. Use of an epitope-specific peptide showed a similar induction of proliferation (data not shown). Similarly, the percentage of CD8+ T cells capable of producing IFN-γ and TNF-α was increased in the presence of the blocking antibodies (data not shown). Identical manipulations had no effect on the proliferation of HIV-specific CD8+ T cells in the absence of T-cell receptor stimulation. These data are in line with recently published work in the murine lymphocytic choriomeningitis virus model.15
Blockade of both PD-1 and 2B4 engagement restores HIV-specific CD8+ T-cell proliferation. (A) Representative data showing expansion of proliferating CD8+ T cells (carboxyfluorescein succinimidyl ester [CFSE] low) in response to stimulation with HIV-1 Gag peptides in the presence or absence of anti-PD-L1 and/or anti-CD48 antibody blockade. (B) Summary data for expansion of HIV-specific CD8+ T cells under conditions of inhibitory pathway blockade (n = 11). Statistical comparisons were conducted using the Friedman test.
Blockade of both PD-1 and 2B4 engagement restores HIV-specific CD8+ T-cell proliferation. (A) Representative data showing expansion of proliferating CD8+ T cells (carboxyfluorescein succinimidyl ester [CFSE] low) in response to stimulation with HIV-1 Gag peptides in the presence or absence of anti-PD-L1 and/or anti-CD48 antibody blockade. (B) Summary data for expansion of HIV-specific CD8+ T cells under conditions of inhibitory pathway blockade (n = 11). Statistical comparisons were conducted using the Friedman test.
Discussion
Recently published data suggest that the manipulation of T cell costimulatory pathways may represent a novel approach to enhance and restore virus-specific CD8+ T-cell responses, especially in the context of persistent infections, such as HIV.7,15,32,33 In this study, we describe the selective increase and simultaneous expression of several coinhibitory receptors on HIV-specific CD8+ T cells. These observations are consistent with recent reports of an active role for multiple coinhibitory receptors in the regulation of virus-specific CD8+ T cells.15,34 Furthermore, the pattern of inhibitory receptor expression was found to correlate with the level of HIV-specific CD8+ T-cell exhaustion; again, this is consistent with previous studies in other systems.15 However, it remains unclear whether this simultaneous expression of inhibitory molecules represents a biologic need for tight regulation of virus-specific CD8+ T-cell responses.
In more detailed analyses, we observed a correlation between cellular differentiation and the expression of PD-1 and CD160; specifically, these inhibitory receptors were expressed predominantly in the CM, TM, and EM compartments. In contrast, the pattern of 2B4 expression was consistently high in all bulk memory populations in samples from HIV+ donors and exhibited a trend for greater expression with greater degrees of differentiation. Previous studies have shown that 2B4 can elicit both “stimulatory” and “inhibitory” signals depending on the cell type, the level of its expression, and the abundance of adaptor molecules.18,35 Whether the expression of 2B4 in different memory compartments is associated with a positive or negative signaling for T-cell activation is not clear and should be addressed in future studies. It is possible that these coexpression patterns define signals that are necessary during CD8+ T maturation, especially within the less differentiated memory compartments (CM, TM, and EM), with each receptor mediating a distinct and determinative cytosolic signal during the maturation process. However, further work is necessary to clarify the function of these individual inhibitory receptors in different memory CD8+ T-cell populations.
The inhibitory receptors examined in this study were expressed to a greater degree on CD8+ T cells from HIV+ persons compared with those from HIV− persons. HIV-specific T-cell receptor-mediated stimulation, general immune activation, and direct functional effects of viral particles/proteins could be among the mechanisms that lead to this pervasive expression pattern.4,36-38 Treatment with ART reduced inhibitory receptor expression, in some cases even to the levels observed on CD8+ T cells from HIV− persons; this is consistent with previously observed effects of ART on CD8+ T-cell functionality.39 It is notable that CD160 expression was significantly higher on naive CD8+ T cells from HIV+ persons compared with the corresponding cells from HIV− persons and that this was reversed by ART. This particular expression pattern could potentially be attributable to the suppression of the general immune activation after ART. We did observe an up-regulation of LAG-3 only on bulk CM CD8+ T cells and not on virus-specific CD8+ T cells. We assume that this change is not related to antigen-specific T-cell receptor stimulation and could be a bystander effect of altered CD8 T-cell dynamics because of highly active antiretroviral therapy.
No correlation was found between CD4 counts and simultaneous expression of PD-1, CD160, 2B4 either before or after ART, indicating that this pattern is not related to CD4 reconstitution. Previous studies have demonstrated a correlation between pVL and PD-1 expression on HIV-specific CD8+ T cells.12,14 In agreement with our previous data,13 we were not able to confirm such a relationship in this study. A strong correlation was observed, however, between pVL and the simultaneous expression of PD-1, CD160, and 2B4 on HIV-specific CD8+ T cells (Figure 4). This association indicates that the simultaneous expression of these inhibitory markers could serve as a more accurate phenotypic composite to demarcate exhausted HIV-specific CD8+ T cells. Furthermore, the reduced expression of these markers after ART was found to be associated with undetectable viral loads, indicating that this expression pattern could also be used to predict viral suppression. More experiments, however, are needed to formally determine whether the expression patterns of these markers can act as a surrogate for full virus suppression.
The importance of the PD-1/PD-L1 pathway in the functional exhaustion of CD8+ T cells in HIV+ persons has been reported by several groups.12-14 However, our previous work revealed no association between PD-1 expression and the ex vivo production of cytokines by virus-specific CD8+ T cells.13 Here we report that the simultaneous expression of several inhibitory receptors was correlated with the ex vivo capacity of both HIV-specific and CMV-specific CD8+ T cells to produce multiple cytokines (Figure 5). Thus, the relative expression of these inhibitory receptors in combination on HIV-specific CD8+ T cells could potentially explain contradictory data in the literature12-14 regarding the role of “exhaustion” molecules in cytokine production by virus-specific CD8+ T cells. Our data also demonstrate that simultaneous coexpression of 3 markers (PD-1, CD160, and 2B4) better defines cytokine-exhausted CD8+ T cells than do any one of those markers individually.
Manipulation of costimulatory molecules has been shown to alter the proliferation/survival capacity of virus-specific CD8+ T cells both in vitro12-14 and in vivo.31 Simultaneous blockade of the PD-1/PD-L1 and 2B4/CD48 interactions in the current study revealed a synergistic effect on the proliferation of HIV-specific CD8+ T cells, thereby indicating that these receptors can function independently, at least in vitro (Figure 6). Such functional independence could be critical in vivo, where inhibitory receptor expression likely varies on different cell populations and at different anatomic sites, and where the expression kinetics and distribution of their respective cognate ligands may also differ.
What are the clinical implications of these findings? Although our data indicate that manipulation of multiple negative regulators of T-cell function may be more effective at restoring T-cell function than approaches targeting individual factors, one must remember that T cells express these negative regulators for a reason, and relieving them from the natural inhibitory effects of these molecules could have detrimental effects on the host. Experimental approaches targeting these negative regulators should first be tested in animal models specifically looking for evidence of unrestrained immune activation or autoimmune phenomena. In addition, short-term, rather than long-term, manipulation of these negative regulators (such as acute therapy to help clear infection or as adjuvants in vaccination) would seem to offer a safer environment in which to test such therapy.
Overall, our data revealed a highly complex expression pattern of inhibitory receptors on HIV-specific CD8+ T cells. Specific combinations of inhibitory receptor expression enable a better definition of exhausted HIV-specific CD8+ T-cell populations and provide information that relates to the immune control of viremia and disease status. Furthermore, these inhibitory receptor-mediated pathways represent potential targets for novel immune therapies in HIV-infected persons.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study subjects for their participation as well as D. Ambrozak, T. Prout, A. Heisler, B. J. Hill, M. F. Quigley, and J. R. Almeida for technical assistance and thoughtful discussions.
This work was supported by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (K24 AI64086; R.H.), the University of California San Diego Center for AIDS Research (AI36214; R.H.), and the San Diego AIDS Clinical Trial Group (CTU AI69432; R.H.). D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow.
National Institutes of Health
Authorship
Contribution: T.Y., C.P., and R.A.K. provided primary conception, execution, and data analysis; D.A.P., J.P.C., G.F., P.D.K., D.C.D., and R.H. contributed to primary conception, interim discussions, and manuscript preparation; M.N. performed the statistical analysis; P.K.C. and M.R. provided expertise in flow cytometry and panel development and supplied in-house conjugated antibodies; E.G. provided the tetramers; T.Y. and C.P. performed experiments and data acquisition; T.Y., D.A.P., C.P., and R.A.K. wrote the paper; and all the coauthors assisted in manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard A. Koup, Immunology Laboratory, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 40 Convent Dr, MSC 3022, Bldg 40, Rm 3502, Bethesda, MD 20892; e-mail: rkoup@mail.nih.gov.

![Figure 6. Blockade of both PD-1 and 2B4 engagement restores HIV-specific CD8+ T-cell proliferation. (A) Representative data showing expansion of proliferating CD8+ T cells (carboxyfluorescein succinimidyl ester [CFSE] low) in response to stimulation with HIV-1 Gag peptides in the presence or absence of anti-PD-L1 and/or anti-CD48 antibody blockade. (B) Summary data for expansion of HIV-specific CD8+ T cells under conditions of inhibitory pathway blockade (n = 11). Statistical comparisons were conducted using the Friedman test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/18/10.1182_blood-2010-11-317297/4/m_zh89991170510006.jpeg?Expires=1767770799&Signature=LsiDzQoCy7FPPWHilXNKnrbK0OUv1lVjpDIEdQGwkPKQLGMuicMbJ7zZNsAhpwRe3GiTK1sdevFbYEKqF54BWSSWC3OyFJzdsqz7TIpKoehnMD2QddJm4ePvv3go0ZG1m2w03GZRQoFlL1mAxxGW43vnWpYsjBDFf-O3PlI8LZ5z3UlG24z13YHK~pQPYAw~OIEKOIBhqFb91RvTfgX7Y0MU4e8Vz6QVfhi1KB6-sAzGqpN5qxtK-3VPv2-9P~JNb8jTutthwiJiF~HtApgzk34tB~qiyoOVkMPEAG900WQd9ib~S~Tx4Hot1Mrb8Alv8OSSr3ecUxcPcNTUCJloJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
