Abstract
COP9 plays a role in plant innate immunity. The role of COP9 in mammalian innate immune responses is unknown. Here, we show that the COP9 signalosome subunit 5 (CSN5) is required for activation of proinflammatory kinases p38 and Erk and for down-regulation of the expression of genes regulated by nuclear factor E2-related factor 2. Mice with myeloid-specific CSN5 deficiency have lower mortality in polymicrobial sepsis. CSN5 is required for both Toll-like receptor (TLR) and reactive oxygen species–mediated deneddylation of Cul3, which is essential for Cul3/Keap1-mediated degradation of nuclear factor E2-related factor 2. On the basis of our results COP9 subunit CSN5 is considered to be an essential component of mammalian innate immunity.
Introduction
Plants, unlike mammals, lack a somatic adaptive immune system. Instead, they rely on innate immunity, and COP9 plays a role in plant innate immune responses.1,2 The role of COP9 in mammalian innate immune responses is unknown. The COP9 signalosome (CSN) is an evolutionarily conserved multisubunit complex (CSN1-CSN8) that regulates the activity of cullin-RING ubiquitin ligases by removing the ubiquitin-like peptide NEDD8.3-5 Among the 8 subunits of the CSN, CSN5 is the most probable component to play a critical role in holo-CSN complex–dependent deneddylase activity.6 CSN5 has been implicated in the functions of cytokine macrophage migration inhibitor factor7 and leukocyte functional antigen 1.8 Little is known about the inflammatory signaling pathways of TLRs that are regulated by CSN5 in macrophages, a cell type central to the initiation of innate immune responses.
TLRs have been shown to play a crucial role in host defense against pathogenic microbes in innate immunity. TLR ligand binding leads to activation of several MAPK pathways that modulate inflammatory responses. The nuclear factor E2-related factor 2 (NRF2) is a master transcriptional activator of genes encoding numerous cytoprotective enzymes that are induced in response to TLR ligand stimuli9-13 and endogenously derived oxidative/electrophilic agents such as reactive oxygen species (ROS).14 However, how NRF2 cross talk with proinflammatory pathways occurs on TLR activation has not been established.
In this study, we sought to examine the function of CSN5 in innate immune responses by conditional ablation of CSN5 gene expression, specifically in myeloid cells. Knockout (KO) of CSN5 in macrophages significantly attenuates the induction of phosphorylated p38 and Erk on TLR ligand stimulation. The attenuation is specific for MAPK p38 and Erk without affecting activation of NF-κB. Depletion of CSN5 in macrophages leads to a marked increase in the regulation of a group of antioxidation and anti-inflammatory genes, including HO-1, which plays a critical role in anti-inflammation.15-17 The up-regulation of HO-1 is correlated with the reduction of Cul3 and enhancement of neddylated Cul3 in macrophages and the accumulation of NRF2. KO of CSN5 also enhanced ROS-mediated neddylation of Cul3. These findings suggest that CSN5 functions to maintain the stability of Cul3 complexed with Keap1 that serves as an E3 complex to degrade NRF2. Maintaining the stability of the Cul3 complex facilitates NRF2 ubiquitination and suppresses anti-inflammation gene expression in macrophages. Collectively, our studies identify for the first time a COP9 subunit CSN5-dependent pathway linking the expression of anti-inflammation and antioxidation genes regulated by NRF2 to the TLR pathway in myeloid cells.
Methods
Generation of myeloid-specific CSN5 KO mice
CSN5/JAB1flox/flox (CSN5fl/fl; provided by Ruggero Pardi, Vita-Salute San Raffaele University, Milan, Italy)18 was generated by introducing a pair of loxP sites into the CSN5 as described.18,19 CSN5-deficient mice were obtained by crossing male mice with loxP-flanked CSN5 with female mice expressing Cre under control of the lysozyme M gene (The Jackson Laboratory). Heterozygous mice were then bred to obtain homozygous KO mice and wild-type littermates for experiments. Genomic DNA was amplified with primers p1 and p3 for CSN5.18 All animal studies were conducted according to protocols approved by the University of Louisville Institutional Animal Care and Use Committee.
Macrophage and dendritic cell culture
Nitric oxide production
Nitric oxide (NO) levels in serum, bronchoalveolar lavage (BAL) fluid, and cultured supernatant fluids were measured with a nitrate and nitrite colorimetric assay kit (Griess Reagent Kit; Invitrogen) according to the manufacturer's instruction.
Antibodies and reagents
Antibodies used for immunoblot analysis were as follows: anti-CSN5 (BIOMOL International, LP); anti-NRF2, anti-Keap1, anti–HO-1, and anti-IκBα (all from Santa Cruz Biotechnology); anti-Flag and anti-Myc (both from Sigma-Aldrich); anti-p38, anti-Erk, antitubulin, and antibody to phosphorylated Erk (9101) and p38 (all from Cell Signaling Technology). Antibodies and other reagents were purchased as specified: anti-Cul3 (Epitomics Inc); zinc protoporphyrin IX (ZnPP) and copper protoporphyrin IX (CuPP; Frontier Scientific Inc); ATP, hydrogen peroxide, diphenylene iodonium chloride (DPI), N-acetyl-L-cysteine (NAC), and cycloheximide (Sigma-Aldrich); poly(I:C), Pam3CSK4 (Ultrapure LPS); CL097 (InvivoGene); and recombinant mouse TNF-α (eBioscience).
Infectious challenge
Age-matched mice were anesthetized by intraperitoneal injection with 100 mg/kg ketamine and 20 mg/kg xylazine. The cecum was exposed by a 1-cm midline incision on the anterior abdomen and subjected to ligation of its distal portion and punctured with 2 passes of a 21-gauge needle (cecum ligation and puncture, CLP). The cecum was then placed back into the abdomen, 1 mL of sterile saline (pyrogen-free 0.9% NaCl) was administrated into the peritoneal cavity, and the incision was closed with the use of 9-mm steel wound clips. Mice were monitored for signs of distress or illness and lethality 3 times daily for 1 week for the survival study. Sera were collected 8 hours after CLP and used to determine TNF-α levels with the use of an ELISA. At day 1 after CLP, 5 mice from each treated group were killed, and the percentage of CD11b+Gr-1+ cells in the peritoneum was determined by FACS analysis. For the determination of bacterial burden in mouse tissues, blood, lung, and liver were collected 48 hours after infection and homogenized, and dilutions of the homogenates were plated on cystine heart agar plates and incubated for 24 hours at 37°C. Bacterial colonies were counted and recorded as CFU per milliliter of blood or per gram of tissue.
Infection of macrophages with Lm or vesicular stomatitis virus
Wild-type Lm (ATCC) was grown overnight with shaking in tryptic soy broth (Difco Laboratories). Vesicular stomatitis virus (VSV) was obtained from Dr Luo Ming (University of Alabama at Birmingham). Macrophages were seeded in 6-well plates at a density of 1 × 106 cells per well in RPMI 1640 serum-free medium and were allowed to attach for 1 hour. Cells were infected for 6-9 hours with Lm (MOI, 10) in 2 mL of RPMI 1640 medium or with VSV (MOI, 5) for 24 hours in 2 mL of RPMI 1640 medium. Culture supernatant fluids were collected for quantification of TNF-α and NO. Cell lysates were used for Western blot analysis of p38, Erk, and Cul3.
BAL fluid preparation and analysis
BAL was performed immediately after anesthesia. The trachea was cannulated with a polyethylene tube through which the lungs were lavaged 3 times with 1.0 mL of PBS (4.0 mL total). The BAL fluid was centrifuged at 150g for 10 minutes, and the supernatant fluids were saved for quantification of TNF-α and NO by ELISA.
Lipopolysaccharide mouse septic shock model and poly(I:C) challenge study of induction of TNF-α and NO
Seven- to 8-week-old CSN5fl/flLyzsM-Cre mice, or wild-type littermates were injected intraperitoneally with lipopolysaccharide (LPS; 30 mg/kg of body weight; Sigma). Mice were monitored for mortality during a period of 7 days. Seven- to 8-week-old CSN5fl/flLyzsM-Cre mice or wild-type littermates were injected intraperitoneally with poly(I:C) (2.5 mg/kg of body weight). Induction of TNF-α and NO in sera and BAL fluid was quantified at 4 and 18 hours, respectively, after the challenge.
Immunohistochemistry
The lung issues were immersed in 10% formaldehyde fixative for 24 hours. The lung lobes were then washed for 8 hours with tap water to remove the formaldehyde. Sections were incubated with a mouse biotin-conjugated anti-myeloperoxidase monoclonal antibody (Abcam). After the primary antibody, the sections were incubated with a streptavidin peroxidase for 5 minutes at room temperature, with tris buffer washes between steps. The substrate diaminobenzidine tetra hydrochloride (DAB) was used for visualization of the antibody-antigen complex. The sections were lightly counterstained with hematoxylin and cover slips were applied following dehydration through graded alcohols to xylene. For each tissue section analyzed, a control slide was incubated with an irrelevant antibody. All sections were evaluated by a Nikon Elipse TE300 microscope (Nikon Inc).
Other biologic effects of the KO of CSN5 in myeloid cells were determined with the use of several techniques that have been described previously.21,22 Details of each method used in this study are described in supplemental Experimental Procedures (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
Statistical significance was calculated with the 2-tailed Student t test. P values of < .05 were considered significant.
Results
Myeloid-specific ablation of CSN5 counteracts polymicrobial sepsis induced by CLP and LPS-induced septic shock
To assess the function of COP9 signalosome CSN5 specifically in myeloid cells, CSN5fl/fl18 mice were crossed with mice in which cDNA-encoding Cre recombinase was inserted into the gene-encoding lysozyme M. Lysozyme M (LyzsM-Cre mice) is specifically expressed in myeloid lineage cells.23 PCR and immunoblot analyses (supplemental Figure 1) and FACS analysis (supplemental Figure 2) showed that CSN5 is a myeloid-specific deficiency. Next, we hypothesized that myeloid CSN5 might contribute to septic shock because published data show that activation of myeloid cells play an important role in the development of septic shock in animal models.15,24 We performed CLP procedures on CSN5fl/flLyzsM-Cre mice and wild-type littermates. Thirteen of 20 CSN5fl/flLyzsM-Cre mice (65%) survived 7 days after CLP ligation, whereas only 20% of the wild-type littermates (5 of 25 mice) survived (Figure 1A) during the same period after CLP. The high lethality was associated with an increase in bacterial counts in the blood, liver, and lung of septic wild-type littermate mice, whereas CSN5fl/flLyzsM-Cre mice had minimal numbers of bacterial CFUs in these tissues (Figure 1B). Sera levels of TNF-α were greater in wild-type littermate mice than in CSN5fl/flLyzsM-Cre mice 8 hours after CLP treatment (Figure 1C). In addition, we observed that neutrophils from CSN5fl/flLyzsM-Cre mice had decreased expression of the activation marker CD11b (Figure 1D). The results of CLP-induced septic shock were further supported by a second LPS-induced septic shock model. Greater than 75% of mice (42 of 55) were protected when CSN5fl/flLyzsM-Cre mice were challenged with LPS (Figure 1E). In contrast < 20% (9 of 50) of the wild-type littermates survived 7 days after an LPS challenge.
KO of myeloid CSN5 leads to enhanced survival after CLP. (A) Survival after 7 days of moderate CLP or sham CLP in 8- to 12-week-old female or male wild-type littermates (CSN5fl/fl, n = 25) mice. Data were pooled from the 2 experiments performed. (B) Bacterial CFUs in lung, blood, and liver 48 hours after CLP. (C) ELISA analysis of plasma TNF-α of littermates or CSN5fl/flLyzsM-Cre mice 8 hours after CLP. (D) Flow cytometric analysis of CD11b+Gr-1+ cells in the peritoneal fluid of littermates or CSN5fl/flLyzsM-Cre mice 24 hours after CLP. Data are expressed as mean ± SEM; *P < .05; **P < .01. (E) Survival after 7 days of LPS-challenged wild-type littermates (CSN5fl/fl n = 50) or CSN5fl/flLyzsM-Cre (n = 55). Data were pooled from the 3 experiments performed. *P < .05; **P < .01.
KO of myeloid CSN5 leads to enhanced survival after CLP. (A) Survival after 7 days of moderate CLP or sham CLP in 8- to 12-week-old female or male wild-type littermates (CSN5fl/fl, n = 25) mice. Data were pooled from the 2 experiments performed. (B) Bacterial CFUs in lung, blood, and liver 48 hours after CLP. (C) ELISA analysis of plasma TNF-α of littermates or CSN5fl/flLyzsM-Cre mice 8 hours after CLP. (D) Flow cytometric analysis of CD11b+Gr-1+ cells in the peritoneal fluid of littermates or CSN5fl/flLyzsM-Cre mice 24 hours after CLP. Data are expressed as mean ± SEM; *P < .05; **P < .01. (E) Survival after 7 days of LPS-challenged wild-type littermates (CSN5fl/fl n = 50) or CSN5fl/flLyzsM-Cre (n = 55). Data were pooled from the 3 experiments performed. *P < .05; **P < .01.
Lung inflammation was significantly reduced in CSN5fl/flLyzsM-Cre mice
Next, lung inflammation was examined. CLP ligation in the wild-type littermates induced significant increases in myeloperoxidase–positive leucocytes infiltrating the lung (Figure 2A). In contrast, CSN5fl/flLyzsM-Cre mice had significantly reduced numbers of leucocytes (Figure 2A). Elevated TNF-α and NO production are characteristic of CLP ligation–induced inflammation in the lung. To determine whether the reduced severity of inflammation observed in CLP-induced sepsis in our septic mouse model was associated with TNF-α and NO production in the lung, TNF-α (Figure 2B) and NO (Figure 2C) quantities were measured in the sera and BAL fluid at 8 and 18 hours, respectively, after CLP ligation. Both TNF-α and NO levels in the sera and lungs of wild-type littermates were higher than in the CSN5fl/flLyzsM-Cre group of mice.
KO of myeloid CSN5 leads to prevention of LPS-induced pulmonary inflammation in CSN5fl/flLyzsM-Cre mice. (A) Immunohistologic staining of lung sections of CSN5fl/fl and CSN5fl/flLyzsM-Cre mice at 48 hours after CLP ligation. Representative antimyeloperoxidase-stained sections (original magnification ×10) of lung are shown 48 hours after the CLP ligation. The induction of TNF-α (B) and NO (C) production in the sera and BAL after CLP ligation was determined by ELISA. (B-C) Data are presented as mean ± SEM. n = 10; **P < .01.
KO of myeloid CSN5 leads to prevention of LPS-induced pulmonary inflammation in CSN5fl/flLyzsM-Cre mice. (A) Immunohistologic staining of lung sections of CSN5fl/fl and CSN5fl/flLyzsM-Cre mice at 48 hours after CLP ligation. Representative antimyeloperoxidase-stained sections (original magnification ×10) of lung are shown 48 hours after the CLP ligation. The induction of TNF-α (B) and NO (C) production in the sera and BAL after CLP ligation was determined by ELISA. (B-C) Data are presented as mean ± SEM. n = 10; **P < .01.
KO of myeloid CSN5 leads to the prevention of p38 and Erk MAPK activation of TLR pathway
Activation of TLR-mediated pathways in myeloid cells plays an important role in the development of septic shock induced by CLP and LPS. However, the expression of genes regulated by CSN5 in TLR ligand–stimulated macrophages is not known. MAPK signaling cascades are regulated by phosphorylation and dephosphorylation on serine or threonine residues or both residues and respond to activation of TLR receptors. To investigate the function of CSN5 in TLR signaling, we examined activation of the MAPK cascades in CSN5-deficient and wild-type mouse macrophages. Macrophages were stimulated with poly(I:C) (Figure 3A), a ligand representative of a viral infection, or LPS (Figure 3B), representative of a bacterial infection.
TLR ligand and TNF-α but not IL-1β induce activation of MAPKs Erk and p38 that depend on CSN5. Immunoblot analysis of phosphorylated p38 and phosphorylated Erk from CSN5fl/fl and CSN5fl/flLyzsM-Cre BMDMs stimulated with poly (I:C) (50 μg/mL) for 60 minutes (A), stimulated with LPS (100 ng/mL) for 30 minutes (B). An α-tubulin blot was included to indicate lane loading. Data are representative of ≥ 5 independent experiments. (C) Immunoblot analysis of p38, Erk, and IκBα from CSN5fl/fl or CSN5fl/flLyzsM-Cre BMDMs stimulated with TNF-α (30 ng/mL) for 15 minutes. Data are representative of ≥ 3 experiments.
TLR ligand and TNF-α but not IL-1β induce activation of MAPKs Erk and p38 that depend on CSN5. Immunoblot analysis of phosphorylated p38 and phosphorylated Erk from CSN5fl/fl and CSN5fl/flLyzsM-Cre BMDMs stimulated with poly (I:C) (50 μg/mL) for 60 minutes (A), stimulated with LPS (100 ng/mL) for 30 minutes (B). An α-tubulin blot was included to indicate lane loading. Data are representative of ≥ 5 independent experiments. (C) Immunoblot analysis of p38, Erk, and IκBα from CSN5fl/fl or CSN5fl/flLyzsM-Cre BMDMs stimulated with TNF-α (30 ng/mL) for 15 minutes. Data are representative of ≥ 3 experiments.
Wild-type macrophages showed an intense activation of MAPK p38 and Erk after poly(I:C) stimulation, as indicated by induction of the phosphorylation of the p38 and Erk that peaked at 30 and 60 minutes, respectively, after stimulation. In contrast, CSN5fl/flLyzsM-Cre macrophages had an impaired phosphorylation of p38 and Erk in response to poly(I:C) stimulation (Figure 3A), although a minimum amount of phosphorylated p38 was detected 15 minutes after stimulation. The activation of MAPK p38 and Erk was also impaired in CSN5fl/flLyzsM-Cre macrophages exposed to LPS (Figure 3B), VSV, and Lm (data not shown), which both (ie, p38 and Erk) function as TLR ligands. NF-κB activation is well established in proinflammatory signaling pathways through TLRs; however, CSN5 deficiency did not seem to affect NF-κB activation mediated by TLRs when CSN5-deficient macrophages were stimulated with poly(I:C) or LPS (data not shown). To further explore the role of CSN5 in TNF-α– and IL-1β–mediated signaling, activation of p38, Erk, and NF-κB in BMDMs from CSN5fl/flLyzsM-Cre and CSN5fl/fl mice was determined. Both p38 and Erk were substantially activated in CSN5fl/fl BMDMs but impaired in CSN5fl/flLyzsM-Cre macrophages after TNF-α (Figure 3C) stimulation. In contrast, NF-κB signaling was not impaired in CSN5fl/flLyzsM-Cre BMDMs (Figure 3C). Unlike TNF-α stimulation, IL-1β stimulation had no effect on the phosphorylation of p38 and Erk1/2 and degradation of IκBα in CSN5fl/flLyzsM-Cre BMDMs (data not shown) in comparison with CSN5fl/fl BMDMs. Collectively, the results obtained with BMDMs showed that genetic ablation of CSN5 strongly impaired TLR- and TNF-α–mediated MAPK signaling.
COP9 signalosome CSN5 is required for the activation of myeloid-derived macrophages
Macrophages produce proinflammatory cytokines in response to a variety of TLR ligands. Thus, we assessed cytokine production by CSN5fl/flLyzsM-Cre mice BM macrophages stimulated with TLR ligands, including pam3CSK4, LPS, poly(I:C), and CL097. BMDMs from CSN5fl/flLyzsM-Cre mice produced much less TNF-α than did those from wild-type littermates on TLR ligand stimulation; in particular, there was a greater reduction of TNF-α production when poly(I:C) ligand was used (Figure 4A). Less TNF-α was released from BMDMs of CSN5fl/flLyzsM-Cre mice on poly(I:C) stimulation, and there was a greater reduction as the concentration of poly(I:C) was increased (Figure 4B). These results were further supported by TNF-α reduction and IFNβ induction data generated from CSN5fl/flLyzsM-Cre BM macrophages infected with Lm and VSV (Figure 4C). These results indicate that CSN5 is important in TLR-induced TNF-α production in macrophages. In addition to induction of TNF-α, CSN5 is also important in TLR-induced NO production in macrophages (Figure 4D). These results collectively show that CSN5 has an important function in innate immune responses.
CSN5 is required for activation of macrophages. (A) Flow cytometric analysis of expression of TNF-α in macrophages activated with Pam3csk4 or LPS for 30 minutes, poly(I:C) for 5 hours, or CL097 for 3 hours. (B) ELISA of TNF-α production in the presence of different concentrations of poly (I:C) 1 day after stimulation. (C) ELISA of TNF-α and IFNβ production after macrophages were infected with Lm or VSV for 24 hours. BMDMs were stimulated with TLR ligands. The supernatant fluids were collected at 48 hours (D) after the stimulation. Induction of NO was determined by a nitrate and nitrite colorimetric assay kit. **P < .01; ***P < .005. Data are representative of 4 experiments (n = 5).
CSN5 is required for activation of macrophages. (A) Flow cytometric analysis of expression of TNF-α in macrophages activated with Pam3csk4 or LPS for 30 minutes, poly(I:C) for 5 hours, or CL097 for 3 hours. (B) ELISA of TNF-α production in the presence of different concentrations of poly (I:C) 1 day after stimulation. (C) ELISA of TNF-α and IFNβ production after macrophages were infected with Lm or VSV for 24 hours. BMDMs were stimulated with TLR ligands. The supernatant fluids were collected at 48 hours (D) after the stimulation. Induction of NO was determined by a nitrate and nitrite colorimetric assay kit. **P < .01; ***P < .005. Data are representative of 4 experiments (n = 5).
KO of myeloid CSN5 results in up-regulation of antioxidation genes
To systematically understand the role of CSN5 in macrophage-mediated inflammation, the global gene expression profiles were examined in macrophages. Distinct categories of mRNAs after LPS and poly(I:C) stimulation were identified that are either increased or decreased relative to wild-type–derived macrophages (supplemental Table 1). Most interesting was the finding that LPS and poly(I:C) stimulation resulted in substantially increased numbers of genes associated with antioxidation and a decreased number of proinflammatory genes in CSN5 KO macrophages. In particular, HO-1 and glutamate cysteine ligase (GCL) were increased significantly. KO of CSN5 in BMDMs led to a marked increase in HO-1 mRNA even without stimulation.
In agreement with the microarray analysis, real-time PCR analysis of macrophages stimulated with different TLR ligands showed a substantial reduction in the amount of these proinflammatory-associated mRNAs and a concomitant increase in the mRNA associated with the antioxidation substrates, including GCLC, GCLM, HO-1, SRXN1, and TXNRD1 (supplemental Figure 3). Some of these real-time PCR results were further confirmed by Western blot analysis of HO-1 (Figure 5A) in macrophages with the use of different stimuli. In addition, the protein level of HO-1 was also markedly increased in unstimulated CSN5fl/flLyzsM-Cre macrophages (Figure 5A lane 1 vs lane 4), indicating that HO-1 is also regulated by CSN5 under unstimulated conditions. Collectively, these data show that CSN5 is important for inhibiting the expression of mRNAs that encode proteins required for the antioxidation and induction of mRNAs that encode proteins required for inflammation after TLR ligand stimulation.
CSN5 inhibits the expression of antioxidation and anti-inflammation genes regulated by NRF2. (A) Western blot analysis of the production of HO-1 protein in CSN5fl/fl or CSN5fl/flLyzsM-Cre macrophages activated with LPS (100 ng/mL) or poly(I:C) (50 μg/mL) for 24 hours. An α-tubulin blot was included to indicate lane loading. Data are representative of 5 experiments. (B) Immunoblot analysis of HO-1 lysates from CSN5fl/flLyzsM-Cre primary peritoneal macrophages transfected with scrambled siRNA or mouse HO-1 siRNA and Western blot analyzed with anti–HO-1 antibodies. An α-tubulin blot was included to indicate lane loading. (C) ELISA of TNF-α production in macrophages transfected with scrambled siRNA or HO-1 siRNA for 24 hours and subsequently activated with poly(I:C) (50 μg/mL) for an additional 24 hours. (D) ELISA of TNF-α production in macrophages treated with poly(I:C) (50 μg/mL), ZnPP (1 μM), ZnPP plus poly(I:C), CuPP (1 μM), or CuPP plus poly(I:C) for 24 hours. Data are representative of 3 experiments or the mean ± SEM of 4 experiments (C-D).
CSN5 inhibits the expression of antioxidation and anti-inflammation genes regulated by NRF2. (A) Western blot analysis of the production of HO-1 protein in CSN5fl/fl or CSN5fl/flLyzsM-Cre macrophages activated with LPS (100 ng/mL) or poly(I:C) (50 μg/mL) for 24 hours. An α-tubulin blot was included to indicate lane loading. Data are representative of 5 experiments. (B) Immunoblot analysis of HO-1 lysates from CSN5fl/flLyzsM-Cre primary peritoneal macrophages transfected with scrambled siRNA or mouse HO-1 siRNA and Western blot analyzed with anti–HO-1 antibodies. An α-tubulin blot was included to indicate lane loading. (C) ELISA of TNF-α production in macrophages transfected with scrambled siRNA or HO-1 siRNA for 24 hours and subsequently activated with poly(I:C) (50 μg/mL) for an additional 24 hours. (D) ELISA of TNF-α production in macrophages treated with poly(I:C) (50 μg/mL), ZnPP (1 μM), ZnPP plus poly(I:C), CuPP (1 μM), or CuPP plus poly(I:C) for 24 hours. Data are representative of 3 experiments or the mean ± SEM of 4 experiments (C-D).
We then determined whether up-regulation of HO-1 leads to the inhibition of TNF-α production in CSN5-deficient macrophages. After initially finding that the HO-1–specific small interfering RNA (siRNA) efficiently inhibited endogenous HO-1 expression in CSN5fl/flLyzsM-Cre macrophages (Figure 5B), we discovered that knockdown of HO-1 reversed the impaired production of TNF-α induced by poly(I:C) in CSN5fl/flLyzsM-Cre macrophages (Figure 5C). To confirm the anti-inflammatory activation of HO-1 involvement in CSN5fl/flLyzsM-Cre macrophages, a specific HO-1 competitive inhibitor, ZnPP was included in experiments. ZnPP caused a significant increase in poly(I:C)–induced TNF-α production in CSN5fl/flLyzsM-Cre macrophages (Figure 5D). In contrast, CuPP, which does not inhibit HO-1, had no effect (Figure 5D). These results collectively show that CSN5 KO causes an increased expression of HO-1 that is responsible for the suppression of production of TNF-α induced by TLR ligands.
CSN5 regulates the stability of Cul3 and degradation of NRF2
It is well established that the expression of HO-1 is regulated by the Cul3/Keap1/NRF2 E3 ligase complex.14,25 NRF2 binds to the antioxidant response element and activates a myriad of genes, including HO-1, that protects cells against oxidative stress and neoplasia.26-29 To elucidate precisely where in the TLR signaling pathways CSN5 inhibits the production of HO-1 and other antioxidative genes regulated by the Cul3/Keap1/NRF2 E3 ligase complex, we examined the stability of the Cul3/Keap1/NRF2 E3 ligase complex located “upstream” of the HO-1 transcription regulator. First, immune precipitation assays showed that CSN5 is coimmunoprecipitated with Cul3 from 293 cells (Figure 6A), presumably because of the interaction of CSN5 with Cul3.30 Ubiquitination of NRF2, measured by immunoprecipitation of NRF2 followed by Western blot analysis of ubiquitin, was attenuated in a dose-dependent manner in 293 cells treated with CSN5 siRNA (Figure 6B). Moreover, knockdown of CSN5 led to accumulation of the full-length NRF2 in nuclear extracts (Figure 6C). NRF2 also accumulated in the nucleus of CSN5fl/flLyzsM-Cre macrophages (Figure 6D). Collectively, these data suggest that CSN5 regulates the ubiquitination of NRF2. We next examined CSN5-mediated cullin deneddylation, which is essential for in vivo–organized E3 functions. KO of CSN5 in BMDMs results in a decrease of unneddylated Cul3 that is accompanied with an increase in neddylated Cul3 (Figure 6E). These data are correlated with a decrease in ubiquitinated NRF2 (Figure 6F) and an increase in the expression of HO-1 (Figure 6G) but without affecting expression of Keap1 (Figure 6F bottom). These results suggest that CSN5 controls the production of functional NRF2 by regulating Keap1/Cul3 E3 ligase–mediated degradation of NRF2 in BMDMs.
CSN5 regulates the ubiquitination of NRF2, a master regulator of expression of antioxidation genes and HO-1. (A) Immunoblot analysis of Cul3-immunoprecipitated lysates from 293 cells treated with MG132 for 6 hours and blotted with anti-CSN5, NRF2, and Keap1. (B) Immunoblot analysis of anti-Flag (NRF2)–immunoprecipitated lysates from 293 cells with the use of anti-HA (ubiquitin) antibody. 293 cells were cotransfected with the plasmids expressing HA-ubiquitin (Ub-HA), Flag-tagged NRF2 (NRF2-Flag), Myc-tagged Cul3 (Cul3-Myc), T7-tagged Keap1 (Keap1-T7), ROC1, and CSN5 siRNA (0, 100, 200, and 300nM) for 24 hours before the cells were harvested for immunoblots. (C) Immunoblot analysis of anti-NRF2 of 293 cell nuclear extracts. 293 cells were transfected with different concentrations of siRNA CSN5 (0, 100, 200, and 300nM) and expression vectors for NRF2-Flag and Cul3-Myc. Total cell lysates were subjected to immunoblot analysis with anti-Flag antibodies. (D) Immunoblot analysis of anti-NRF2 from the nuclear extracts of CSN5fl/flLyzsM-Cre BMDMs. Data are representative of 3 independent experiments (C-D). (E) Immunoblot analysis of Cul3 in wild-type or CSN5 KO BMDMs. (F) Immunoblot analysis of NRF2-immunoprecipitated lysates of macrophages with the use of antipolyubiquitin or anti-Keap1 antibodies. (G) Immunoblot analysis of HO-1 in wild-type or CSN5 KO BMDMs. An α-tubulin blot was included to indicate lane loading. (H) Immunoblot analysis of Cul3 in 293 cells transfected with the different concentrations of siRNA CSN5 (0, 100, 200, and 300nM) for 24 hours. Data are representative of 3 experiments. An α-tubulin blot was included to indicate lane loading. Data are representative of 3 experiments (E-G).
CSN5 regulates the ubiquitination of NRF2, a master regulator of expression of antioxidation genes and HO-1. (A) Immunoblot analysis of Cul3-immunoprecipitated lysates from 293 cells treated with MG132 for 6 hours and blotted with anti-CSN5, NRF2, and Keap1. (B) Immunoblot analysis of anti-Flag (NRF2)–immunoprecipitated lysates from 293 cells with the use of anti-HA (ubiquitin) antibody. 293 cells were cotransfected with the plasmids expressing HA-ubiquitin (Ub-HA), Flag-tagged NRF2 (NRF2-Flag), Myc-tagged Cul3 (Cul3-Myc), T7-tagged Keap1 (Keap1-T7), ROC1, and CSN5 siRNA (0, 100, 200, and 300nM) for 24 hours before the cells were harvested for immunoblots. (C) Immunoblot analysis of anti-NRF2 of 293 cell nuclear extracts. 293 cells were transfected with different concentrations of siRNA CSN5 (0, 100, 200, and 300nM) and expression vectors for NRF2-Flag and Cul3-Myc. Total cell lysates were subjected to immunoblot analysis with anti-Flag antibodies. (D) Immunoblot analysis of anti-NRF2 from the nuclear extracts of CSN5fl/flLyzsM-Cre BMDMs. Data are representative of 3 independent experiments (C-D). (E) Immunoblot analysis of Cul3 in wild-type or CSN5 KO BMDMs. (F) Immunoblot analysis of NRF2-immunoprecipitated lysates of macrophages with the use of antipolyubiquitin or anti-Keap1 antibodies. (G) Immunoblot analysis of HO-1 in wild-type or CSN5 KO BMDMs. An α-tubulin blot was included to indicate lane loading. (H) Immunoblot analysis of Cul3 in 293 cells transfected with the different concentrations of siRNA CSN5 (0, 100, 200, and 300nM) for 24 hours. Data are representative of 3 experiments. An α-tubulin blot was included to indicate lane loading. Data are representative of 3 experiments (E-G).
It has been shown that neddylated Cul3 is unstable.31 CSN deneddylation recycles the unstable neddylated cullins into stable unneddylated cullins and promotes cullin-organized E3 activity in vivo. Therefore, we measured the protein stability of neddylated Cul3 in 293 cells that were treated with cycloheximide to block protein synthesis. With the knockdown of CSN5, the unneddylated Cul3 protein decreased in a siRNA CSN5 dose-dependent manner (Figure 6H). This result suggests that CSN5-mediated neddylation/deneddylation of Cul3 in Cul3/Keap1/NRF2 complex is a prominent mechanism in regulating HO-1 production.
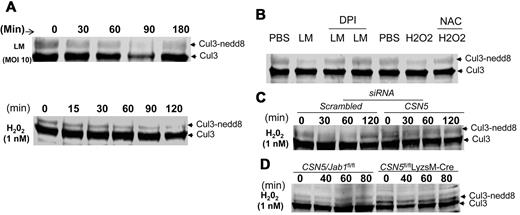
On the basis of a recent finding32 that ROS regulates Cul1 deneddylation and CSN5 regulates Cul3 neddylation (as shown in Figure 6), we sought to determine whether neddylation of Cul3 is regulated by CSN5 cross talk with the ROS pathway. First, we analyzed whether ROS induced by bacterial infection regulates Cul3 deneddylation. On Lm infection, neddylated Cul3 decreased during a period of 60 minutes and was undetectable at 90 minutes; however, by 180 minutes the presence of Cul3 was fully recovered (Figure 7A top). This result was reproduced when hydrogen peroxide was used as a stimulus (Figure 7A bottom). To examine the possible involvement of ROS, we monitored Lm or hydrogen peroxide–induced Cul3 deneddylation in 293 cells in the presence of DPI, which prevents bacterially induced increases in ROS, or NAC, an antioxidant reagent. Pretreatment with DPI impaired the deneddylation of Cul3 induced by Lm (Figure 7B), and NAC also blocked hydrogen peroxide–induced Cul3 deneddylation (Figure 7B). These results suggest that Cul3 neddylation is mediated by ROS production. Given that deneddylation of Cul3 is regulated by the COP9 signalosome (CSN) as shown in Figure 6, we then examined Cul3 deneddylation induced by oxidative stress in CSN5-deficient cells. Knockdown of CSN5 in 293 cells exhibited a stronger neddylation of Cul3 after hydrogen peroxide stimulation than in 293 cells transfected with control siRNA (Figure 7C). This result was reproducible when BMDMs from CSN5fl/flLyzsM-Cre mice were used (Figure 7D). These data collectively indicate that Cul3 deneddylation induced by ROS depends on the COP9 subunit CSN5.
CSN5 has an effect on ROS-mediated deneddylated/neddylated Cul3. (A) Immunoblot analysis of Cul3 from 293 cells infected with Lm for 180 minutes (top) or treated with hydrogen peroxide (H2O2) for 120 minutes (bottom). (B) Immunoblot analysis of Cul3 from 293 cells infected with Lm (MOI, 10) or treated with H2O2 (1mM) in the presence or absence of diphenylene iodonium chloride (DPI; 40 μM) or NAC (20 mM) 30 minutes before the treatments. (D) Immunoblot analysis of Cul3 from 293 cells transfected with scrambled siRNA or CSN5 (100 nM) for 24 hours and subsequently treated with H2O2 for 120 minutes. (D) Immunoblot analysis of Cul3 from CSN5fl/fl or CSN5fl/flLyzsM-Cre BMDMs treated with H2O2 for 80 minutes. Data are representative of 5 (A-D) experiments.
CSN5 has an effect on ROS-mediated deneddylated/neddylated Cul3. (A) Immunoblot analysis of Cul3 from 293 cells infected with Lm for 180 minutes (top) or treated with hydrogen peroxide (H2O2) for 120 minutes (bottom). (B) Immunoblot analysis of Cul3 from 293 cells infected with Lm (MOI, 10) or treated with H2O2 (1mM) in the presence or absence of diphenylene iodonium chloride (DPI; 40 μM) or NAC (20 mM) 30 minutes before the treatments. (D) Immunoblot analysis of Cul3 from 293 cells transfected with scrambled siRNA or CSN5 (100 nM) for 24 hours and subsequently treated with H2O2 for 120 minutes. (D) Immunoblot analysis of Cul3 from CSN5fl/fl or CSN5fl/flLyzsM-Cre BMDMs treated with H2O2 for 80 minutes. Data are representative of 5 (A-D) experiments.
Discussion
The present study showed that COP9 subunit CSN5 is a critical host factor for mounting an appropriate innate immune response, which can determine survival during septic shock. Disruption of CSN5 in mice caused less susceptibility to septic shock induced by bacterial infection. Our work further identifies a previously undescribed COP9 CSN5 function that is essential for innate immune activation of macrophages in terms of TLR-mediated induction of proinflammatory cytokines. Notably, TLR-mediated pathways cross talk with NRF2 through the COP9 subunit, CSN5, which regulates Keap1/Cul3-mediated degradation of NRF2.
As noted previously,14,33 Keap1/Cul3/NRF2 is a master regulator of genes related to anti-inflammatory and antioxidation pathways. Our observation supports the idea that the COP9 subunit CSN5 is a positive regulator of Keap1/Cul3-mediated degradation of NRF2. Furthermore, CSN5-mediated neddylated and deneddylated Cul3 regulates both induction of inflammatory cytokines and inhibition of the anti-inflammation and antioxidation genes regulated by NRF2 in macrophages.
Not only does CSN5 regulate dennedylation of Cul3 on TLR stimulation, but it is also required for Cul3 dennedylation mediated by ROS released from activated macrophages. Our data indicate that hydrogen peroxide–induced reduction of neddylated Cul3 was prevented in CSN5 KO macrophages, suggesting that CSN5 also regulates ROS-induced neddylated Cul3 in a TLR stimulation–independent manner. These findings suggest that CSN5 regulates both TLR-dependent and ROS-mediated Cul3 neddylation/dennedyaltion. KO of CSN5 in macrophages leads to preventing the cycling of neddylation/deneddylation of Cul3 and accumulation of neddylated Cul3 and a reduction of deneddylated Cul3. The prevention of degradation of NRF2 further leads to a loss of regulation of several antioxidation and anti-inflammation genes.
CSN5 is not only required for the suppression of NRF2 regulated anti-inflammatory genes but also is critically involved with the activation of TLR-mediated activation of MAPK p38 and Erk. Our results showed that KO of CSN5 in myeloid cells resulted in the inhibition of the activation of p38 and Erk after poly(I:C) or LPS treatment or bacterial or viral infection.
Taken together, the results presented in this study imply that once macrophages receive proinflammatory stimulation (eg, a bacterial or viral infection), COP9 subunit CSN5 positively up-regulates the innate immune response by activation of MAPK-mediated pathways. Meanwhile, CSN5 negatively regulates anti-inflammatory pathways, such as the NRF2-mediated anti-inflammatory response. It is speculated that for host cells to initiate an inflammatory response in a time-related fashion, both down-regulation of NRF2-mediated anti-inflammation and antioxidation and initiation of MAPK-mediated activation proinflammatory responses are required simultaneously. Perhaps, NRF2 may also be inactivated by a CSN5-dependent activation of p38 and Erk by phosphorylating NRF2 in situations in which Keap1/Cul3-dependent degradation of the NRF2 pathway is not acted on in time.
It is conceivable that the CSN5 subunit of COP9 may be a possible therapeutic target for treatment of many diseases associated with chronic inflammation, including cancers, aging-related diseases because both inflammation and oxidation are crucial mediators for both diseases. This speculation is supported by recent data indicating that CSN5 is dysregulated in both different types of cancers and aging-related diseases.5,34-39
Although we have identified a role for the CSN5 subunit of COP9 in TLR-mediated innate immune responses of macrophages, further research is needed to understand why KO of CSN5 leads to preferential prevention of activation of p38 and Erk1/2 but not of NF-κB in macrophages and also how these activities impinge on the overall physiology of the organism.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Manabu Furukawa (University of Nebraska) for the DNA expression vectors and Dr Luo Ming (University of Alabama at Birmingham) for providing the vesicular stomatitis virus. We thank Dr Jerald Ainsworth for editorial assistance.
This work was supported by the Louisville Veterans Health Administration Medical Center (merit review grants; H.-G.Z.) and by a grant from the Susan G. Komen Breast Cancer Foundation.
Authorship
Contribution: Z.D. and H.-G.Z. designed the research, analyzed and interpreted data, and drafted the manuscript; R.P. provided CSN5fl/fl mice and interpreted findings; X.X. performed cDNA and RT-PCR analysis and interpreted data; and S.V.S., W.G., S.Z., W.C., D.M., and J.M. contributed to drafting the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Huang-Ge Zhang, James Graham Brown Cancer Center, University of Louisville, CTRB 309, 505 Hancock St, Louisville, KY 40202; e-mail: h0zhan17@louisvile.edu; or Zhongbin Deng, James Graham Brown Cancer Center, University of Louisville, CTRB 309, 505 Hancock St, Louisville, KY 40202; e-mail: z0deng01@louisville.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal