In this issue of Blood, MacQarrie and colleagues show that histidine-rich glycoprotein (a negative acute-phase reactant), inhibits activated factor XII, a clotting protein implicated in inflammation and thrombosis.1
Of the 2 mechanisms for triggering blood clotting, only the tissue factor pathway is essential for normal hemostasis. The other—the contact pathway—appears to have no hemostatic role, because patients with severe factor XII deficiency have no bleeding diatheses. On the other hand, several recent in vivo studies have shown that knocking out the factor XII gene or administering factor XIIa inhibitors strongly protects experimental animals from thrombotic and inflammatory challenge.2 Together with a large body of work on the kallikrein/kinin system,3 these fascinating studies have focused renewed attention on the contact pathway, and on factor XII in particular, as a potential target for antithrombotic/anti-inflammatory agents with little danger of inducing hemorrhage. How the contact pathway is activated in vivo has been something of a mystery for many years, because its best known triggering agents (things like glass, kaolin, and diatomaceous earth) are clearly nonphysiologic. We recently showed that inorganic polyphosphate, a highly anionic polymer that accumulates in microorganisms and is secreted from activated platelets, is a potent trigger of the contact pathway and may be the long-sought (patho)physiologic activator of factor XII.4,5 Much remains to be investigated regarding how factor XII activity is regulated in vivo, but the findings of MacQuarrie et al show us an intriguing new mechanism for controlling factor XII activation, potentially connecting it to other molecular players in inflammation, infection, angiogenesis, and thrombosis.6
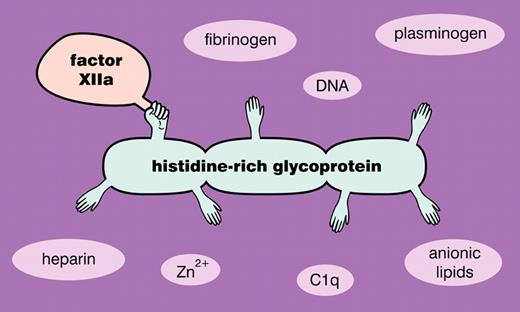
Initiation of the contact pathway of clotting classically involves the autoactivation of factor XII on a suitable surface, as well as the reciprocal activation of plasma prekallikrein by factor XIIa and factor XII by kallikrein. The resulting burst of factor XIIa then activates factor XI to XIa, leading to propagation of the clotting cascade, while the newly generated kallikrein cleaves high molecular weight kininogen to release the vasoactive peptide bradykinin. (In some circumstances, subsets of these reactions can be triggered—with proinflammatory consequences—while not progressing all the way to fibrin formation.7 ) The major plasma inhibitor of the triggering phase of the contact pathway is C1 esterase inhibitor, a serpin that inactivates factor XIIa and plasma kallikrein, albeit with rather slow kinetics. MacQarrie and colleagues now report that histidine-rich glycoprotein binds with high affinity to factor XIIa (but not to zymogen factor XII), especially in the presence of Zn2+ ions.1 Furthermore, histidine-rich glycoprotein strongly inhibits polyphosphate-mediated factor XII autoactivation as well as factor XI activation by factor XIIa, while having only modest effects on the activation of plasma prekallikrein by factor XIIa. These findings therefore implicate histidine-rich glycoprotein, a relatively abundant plasma protein, as a physiologically significant modulator of factor XII activity (see figure).
Histidine-rich glycoprotein interacts with such a wide variety of protein targets that it has been called the Swiss Army knife of mammalian plasma.6 Its many ligands include heparin, DNA, anionic phospholipids, fibrinogen, plasminogen, immune complexes, heme, Zn2+, C1q, and now, factor XIIa. Its ability to bind many of these ligands simultaneously may allow it to function as an adaptor molecule, and it has been implicated in such diverse pathophysiologic roles as phagocytosis of apoptotic and necrotic cells; neutralizing endotoxin and killing microbes; regulating angiogenesis, cell adhesion, and cancer progression; and modulating coagulation and fibrinolysis.6 Knockout mice deficient in histidine-rich glycoprotein have enhanced blood clotting and fibrinolysis.8 They also exhibit increased susceptibility to Streptococcus pyogenes infection, and interestingly, plasma clots formed in the absence of histidine-rich glycoprotein have decreased ability to trap and kill bacteria.9 The fact that histidine-rich glycoprotein is a negative acute phase reactant while fibrinogen, its major plasma ligand, is a positive acute phase reactant suggests that the ability of histidine-rich glycoprotein to put the brakes on factor XII will be diminished during acute phase responses, a condition that could therefore contribute to increased proinflammatory (and prothrombotic?) phenotypes.
Conflict-of-interest disclosure: The author declares no competing financial interest. ■
REFERENCES
National Institutes of Health