Abstract
CD4+ natural killer T (NKT) cells, along with CD4+CD25+ regulatory T cells (Tregs), are capable of controlling aberrant immune reactions. We explored the adoptive transfer of highly purified (> 95%) CD4+NKT cells in a murine model of allogeneic hematopoietic cell transplantation (HCT). NKT cells follow a migration and proliferation pattern similar to that of conventional T cells (Tcons), migrating initially to secondary lymphoid organs followed by infiltration of graft-versus-host disease (GVHD) target tissues. NKT cells persist for more than 100 days and do not cause significant morbidity or mortality. Doses of NKT cells as low as 1.0 × 104 cells suppress GVHD caused by 5.0 × 105 Tcons in an interleukin-4 (IL-4)–dependent mechanism. Protective doses of NKT cells minimally affect Tcon proliferation, but cause significant reductions in interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) production by donor Tcons and in skin, spleen, and gastrointestinal pathology. In addition, NKT cells do not impact the graft-versus-tumor (GVT) effect of Tcons against B-cell lymphoma-1 (BCL-1) tumors. These studies elucidate the biologic function of donor-type CD4+NKT cells in suppressing GVHD in an allogeneic transplantation setting, demonstrating clinical potential in reducing GVHD in HCT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a successful therapy for both malignant and nonmalignant diseases. Immunologic reactions are central to the beneficial effects of HCT, resulting in engraftment and disease control; however, a major limitation of HCT is acute graft-versus-host disease (GVHD), characterized by immunodysregulation and injury of tissues including the skin, gastrointestinal tract, and liver.1 Much work has focused on the use of CD4+CD25+ regulatory T cells (Tregs) in the control of GVHD, and adoptive transfer of Tregs can prevent or ameliorate GVHD while preserving the graft-versus-tumor (GVT) effect.2-9 Whereas multiple mechanisms have been proposed, the net effect is that Tregs consistently suppress the alloreactive T-cell proliferation critical for GVHD induction and disease progression.
Natural killer T (NKT) cells, which express NK and T-cell markers, have also shown the potential to regulate immune reactions. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation and depletive anti–T-cell antibodies correspond with an increase in host-type DX5+TCRαβ+ NKT cells.10 Furthermore, marrow-derived NK1.1+ T cells ameliorate GVHD in an interleukin-4 (IL-4)–dependent manner.11 This concept has been translated to the clinical setting, with a very low rate of acute GVHD and transplant-related mortality.12,13 Other studies have found that invariant NKT cells, expanded in vitro with the ligand α-GalCer, prolonged survival in allogeneic transplantation models, as could administration of α-GalCer.14,15 In contrast, using whole bone marrow from a variety of knockout mice, Kim et al reported that bone marrow type II NKT cells (rather than invariant NKT) cells suppressed GVHD and prolonged survival in mice by mechanisms involving both interferon-γ (IFN-γ) and IL-4 production by bone marrow type II NKT cells.16
In this study, we examined the migration, proliferation, and regulatory capability of unmanipulated donor-type CD4+NKT cells in a major histocompatibility complex (MHC)–mismatched murine model of GVHD. This is the first study to examine the effect of unmanipulated (ie, not stimulated in vitro with α-GalCer) donor NKT cells consisting of both invariant and noninvariant populations. Furthermore, whereas previous studies transferred total invariant NKT cells, we chose to examine CD4+NKT cells because they have been shown to produce much more IL-4 than CD4−NKT cells.17-22 We noninvasively and longitudinally monitored the in vivo trafficking properties of CD4+NKT cells and evaluated their ability to suppress GVHD. We found that administration of small numbers of NKT cells (in relation to the GVHD-inducing conventional T cells) significantly reduced GVHD-related tissue damage and improved survival. We examined the mechanism of NKT-cell–mediated suppression of GVHD and the effect of NKT cells on the GVT effect of HCT.
Methods
Mice
Experiments used gender-matched mice between 7 and 10 weeks of age. BALB/c (H-2d), C57BL/6 (H-2b, CD45.2), IL-4−/− (B6.129P2-Il4tm1Cgn/J), and IFN-γ−/− (B6.129S7-Ifngtm1Ts/J) mice were purchased from The Jackson Laboratory. Luciferase-expressing (luc+) FVB/N L2G85 and C57Bl/6 (H-2b, CD45.1, luc+) mice were generated as described previously.9,23 Animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
Cell isolation
For the isolation of CD4+NKT cells, spleens were processed in phosphate-buffered saline (PBS; Invitrogen) with 2% fetal calf serum (FCS; Invitrogen) into single-cell suspensions. For lymphocyte isolation from liver, livers were perfused with PBS, processed, centrifuged at 800g for 10 minutes, resuspended in 33% isotonic Percoll (GE Healthcare), and centrifuged at 800g for 20 minutes. The pellet was red blood cell lysed, pooled with splenocytes, blocked with FcR block (Miltenyi Biotec), enriched with anti-DX5 MACS beads (Miltenyi Biotec), stained with DX5, T-cell receptorβ (TCRβ), and CD4, and sorted on a FACSAria or FACSAria II flow cytometer (BD Biosciences). CD4/CD8 conventional T cells (Tcons) were prepared from splenocytes and peripheral lymph node cells and enriched with anti-CD4 MACS and then anti-CD8 MACS. Purity was determined by fluorescence-activated cell sorting (FACS) on an LSRII or FACSAria flow cytometer (BD Biosciences), and cells were mixed so that the CD4:CD8 ratio was 2:1. T cell–depleted bone marrow (TCD-BM) was prepared by flushing bones and depleting T cells with anti-Thy1.2 MACS. Tregs were prepared from pooled spleens and lymph nodes, staining for CD25-allophycocyanin and CD4, enriching with anti-allophycocyanin MACS, and sorting for CD4+CD25hi cells on a FACSAria or FACSAria II flow cytometer (BD Biosciences).
Flow cytometric analysis
PBS-57–loaded mCD1d was from the National Institutes of Health Tetramer Facility. The following antibodies were purchased from BD Pharmingen, eBioscience, or BioLegend: CD4 (GK1.5), CD8 (53-6.7), CD45.1 (A20), CD45.2, (104) Thy-1.1(H1S51), Thy-1.2 (53-2.1), CD25 (PC61), H-2Kb (AF6-88.5), H-2Kd (34-2-12), Foxp3 (FJK-16s), IL-2 (JES6-5H4), IL-4 (11B11), IL-5 (TRFK5), IL-10 (JES5-16E3), IL-17 (TC11-18H10.1), tumor necrosis factor-α (TNF-α; MP6-XT22), and IFN-γ (XMG1.2). Foxp3 staining was performed with the anti–mouse/rat Foxp3 Staining Set (eBiosciences). To stain dead cells, propidium iodide was used for freshly isolated cells and ethidium monoazide (Molecular Probes) for fixed cells. Analysis was performed on a FACSAria or LSRII flow cytometer (BD Biosciences).
Intracellular cytokine staining
Cells were stimulated with phorbol myristate acetate (40 ng/mL), ionomycin (2μM), and monensin (2μM; Sigma-Aldrich) for 5 hours at 37°C and 5% CO2 in complete RPMI with 10% FCS, 2mM l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin (Invitrogen), and 5 μg/mL of 2-mercaptoethanol (Sigma-Aldrich). Unstimulated controls were incubated in complete RPMI only. After staining surface antigens with ethidium monoazide, cells were fixed and permeabilized (BD Biosciences) and assessed for IL-2, IL-4, IL-5, IL-10, IL17, IFN-γ, or TNF-α.
Transplantation model to evaluate GVHD and GVT
Balb/c recipients were treated with total body irradiation (200 Kv X-ray source), consisting of 2 doses of 4.2 Gy 4 hours apart. 5 × 106 TCD-BM (C57BL/6) was injected, via tail vein, on day 0, followed by 5 × 105 WT or luc+Tcons (C57BL/6) on day 2. NKT cells were given on day 0 from wild-type (WT), IL-4−/−, or IFN-γ−/− C57BL/6 mice, or, for trafficking, from luc+C57BL/6 mice. Transplanted animals were kept in autoclaved cages with antibiotic water (sulfamethoxazole-trimethoprim; Schein Pharmaceutical). To investigate GVL activity, we used a B-cell lymphoma-1 (BCL-1) model7,24 expressing the luciferase gene to visualize the tumor cells.24 3 × 103luc+BCL1 cells were injected intravenously on day −7 before HCT into Balb/c recipients. Tumor engraftment was verified by bioluminescence imaging (BLI) before total body irradiation. After transplantation, tumor burden was assessed by BLI.
In vivo and ex vivo BLI
BLI was performed as described previously24 with an IVIS 7, IVIS 29, or IVIS Spectrum charge-coupled device imaging system (Xenogen). Images were analyzed with Living Image Software 2.5 (Xenogen) and Igor Pro Carbon Version 4.09 (Wavemetrics).
Histopathology
Tissues were fixed in 10% neutral buffered formalin, and 4-micron–thick, formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin and evaluated by an experienced pathologist (R.H.L.), who was blinded to the experimental groups. Stained tissue sections were evaluated with an Olympus BX-41 microscope with a Canon Powershot G9 camera and Zeiss AxioVision 4.7 digital imaging software.
Statistical analysis
Differences in animal survival (Kaplan-Meier survival curves) were analyzed with the log-rank test. All other comparisons were performed with the 2-tailed Student t test, and P < .05 was considered statistically significant.
Results
NKT cells follow a migration pattern typical of conventional T cells but do not cause GVHD
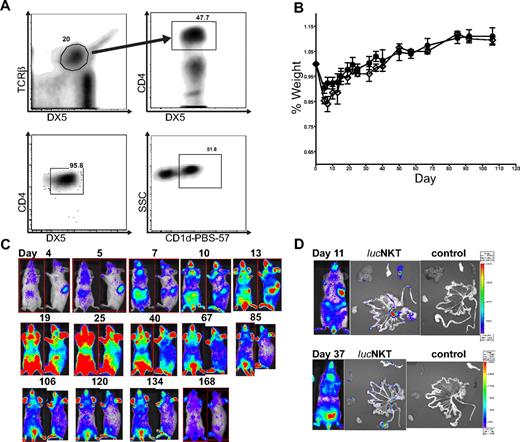
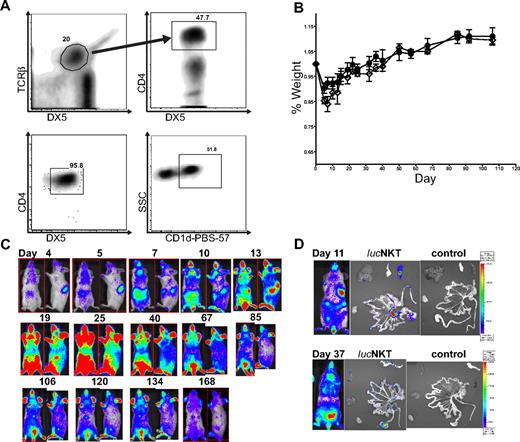
We examined the migration and proliferation of donor-type luc+NKT cells in a transplantation setting. CD4+NKT cells were purified from pooled spleen and liver cells to > 95% purity (Figure 1A). NKT cells were approximately 50% positive for the CD1d–PBS-57 tetramer, indicating that the purified cells included both invariant and noninvariant NKT cells. To observe the proliferation and migratory behavior of NKT cells after allogeneic transplantation, we transferred 5.5 × 105 highly purified (> 95%) NKT cells (DX5+TCRβ+CD4+) from luciferase-positive (luc+) C57BL/6 (H-2b) mice into lethally irradiated Balb/c (H-2d) recipients, together with 5 × 106 TCD-BM from WT C57BL/6 mice, and monitored the animals by BLI. Mice receiving the NKT cells showed no evidence of GVHD or other signs of morbidity or mortality, as illustrated by the fact that the weight curve of mice receiving 5.5 × 105 NKT cells in addition to 5 × 106 TCD-BM was identical to that of mice receiving TCD-BM only (Figure 1B). BLI analysis showed localization of NKT cells in the spleen and lymph nodes by day 4 after transfer, and between days 7 and 10, NKT cells had migrated to the skin (Figure 1C). The total photons emitted peaked around day 25 after transplantation, followed by a steady decline. NKT cells persisted in observable numbers for more than 160 days. Ex vivo BLI imaging indicated that early BLI signals were primarily from the spleen and lymph nodes, and at later time points NKT cells migrated into GVHD target organs such as the gastrointestinal tract and liver (Figure 1D). These results indicate that NKT cells themselves do not cause any observable morbidity or mortality and that they follow a similar migration pattern to that of CD4 or CD8 Tcons.25
NKT cells traffic to GVHD priming sites and target organs but do not cause GVHD. (A) NKT cells were sorted to high purity (> 95%) from C57BL6 donors (H-2b) by first gating TCRβ+DX5+ then CD4+ cells; analysis of sorted cells indicates a population that is approximately 50% positive for PBS-57–loaded CD1d (National Institutes of Health). (B) 5.5 × 105luc+NKT cells transferred into lethally irradiated allogeneic BALB/c (H-2d) mice caused no significant alteration in weight profile compared with mice receiving T cell–depleted C57BL/6 bone marrow (TCD-BM) only. Solid line (●) indicates TCD-BM; dashed line (◇) indicates 550K luc+NKT. Bars indicate means ± SE (n = 3). (C) BLI indicated that NKT cells first appear in the spleen and lymph nodes, followed by migration to the skin and other GVHD target organs. Total photons emitted peaked near day 25 after transplantation, followed by a steady decline. Results shown are from a representative mouse of 3 from 1 of 4 independent experiments. Ventral and lateral images of the same mouse are shown with the day after transplant indicated. (D) Ex vivo imaging of internal organs indicates NKT cells trafficking to the spleen and mesenteric lymph nodes at day 11 and infiltration into the gastrointestinal tract, pancreas, and liver tissue by day 37. Images from mice that did not receive any luc+NKT cells are shown as a control.
NKT cells traffic to GVHD priming sites and target organs but do not cause GVHD. (A) NKT cells were sorted to high purity (> 95%) from C57BL6 donors (H-2b) by first gating TCRβ+DX5+ then CD4+ cells; analysis of sorted cells indicates a population that is approximately 50% positive for PBS-57–loaded CD1d (National Institutes of Health). (B) 5.5 × 105luc+NKT cells transferred into lethally irradiated allogeneic BALB/c (H-2d) mice caused no significant alteration in weight profile compared with mice receiving T cell–depleted C57BL/6 bone marrow (TCD-BM) only. Solid line (●) indicates TCD-BM; dashed line (◇) indicates 550K luc+NKT. Bars indicate means ± SE (n = 3). (C) BLI indicated that NKT cells first appear in the spleen and lymph nodes, followed by migration to the skin and other GVHD target organs. Total photons emitted peaked near day 25 after transplantation, followed by a steady decline. Results shown are from a representative mouse of 3 from 1 of 4 independent experiments. Ventral and lateral images of the same mouse are shown with the day after transplant indicated. (D) Ex vivo imaging of internal organs indicates NKT cells trafficking to the spleen and mesenteric lymph nodes at day 11 and infiltration into the gastrointestinal tract, pancreas, and liver tissue by day 37. Images from mice that did not receive any luc+NKT cells are shown as a control.
Low doses of NKT cells have a protective effect against GVHD
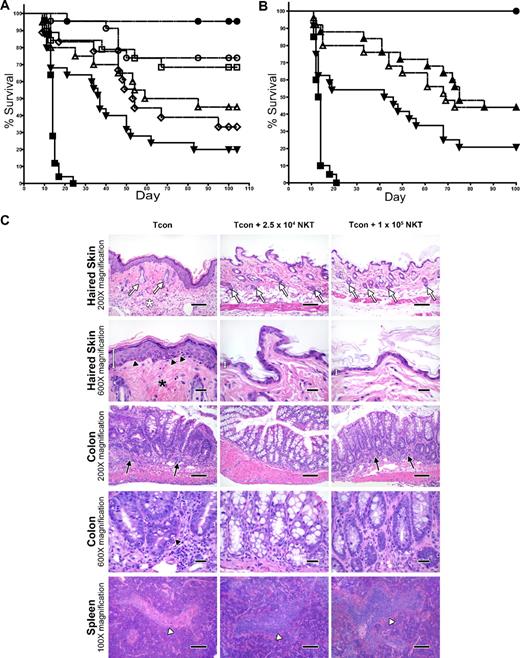
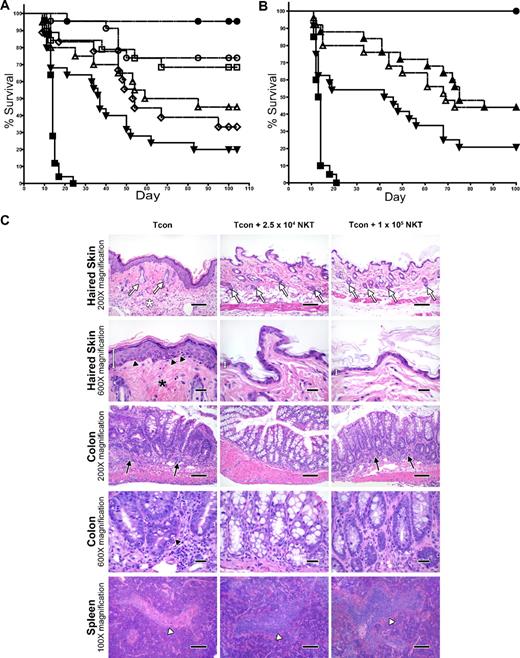
To assess the impact of donor-type NKT cells on GVHD induction by Tcons, we cotransferred various doses of highly purified WT NKT cells from C57BL/6 mice on day 0 with 5 × 106 TCD-BM, followed by 5 × 105luc+Tcons on day 2 into irradiated allogeneic Balb/c recipients. In the absence of NKT cells, the mice developed GVHD, with only 20% of them surviving past 100 days (Figure 2A). However, the addition of 2.5 × 104 NKT cells improved survival to 74% (P = .002 compared with the Tcon-only group). Improvement in survival was also observed with a dose of 1.0 × 104 NKT cells, with 68% of mice surviving beyond 100 days (P = .006 compared with the Tcon-only group). Interestingly, increasing the dose of NKT cells to 5 × 104 or 1 × 105 cells per animal resulted in lesser reduction of GVHD than with the lower NKT cell doses, with only 33% and 45% of mice surviving, respectively. Therefore, the doses of 5 × 104 and 1 × 105 cells did not significantly improve survival over the Tcon-only group (P > .05). In addition, the optimal dose of NKT cells, 2.5 × 104, demonstrated better survival than the dose of 1.0 × 105 NKT cells, with borderline significance (P = .05)
Adoptively transferred donor-type NKT cells protect against GVHD. (A) Doses of NKT cells (DX5+TCRβ+CD4+) from C57BL6 donors (H-2b) were adoptively transferred into lethally irradiated BALB/c mice (H-2d) that had TCD-BM and 5.0 × 105 Tcons (to induce GVHD). Significant improvement in survival was observed with the transfer of either 1.0 × 104 or 2.5 × 104 NKT cells (P < .01). Data shown in graph were pooled from 4 independent experiments. Groups: irradiation control (no BMT; ■; n = 25); TCD-BM only (●; n = 25); 5 × 105 Tcons (▾; n = 25); Tcons + 1 × 105 NKT (▵; n = 20); Tcons + 5 × 104 NKT (◇; n = 18); Tcons + 2.5 × 104 NKT (○; n = 23); and Tcons + 1 × 104 NKT (□; n = 19). (B) Further sorting of NKT cells into PBS-57 CD1d–PBS-57 tetramer-positive and tetramer-negative subpopulations indicates that both invariant and noninvariant NKT cells provide protection against GVHD (P < .05). Graph contains data pooled from 4 independent experiments (n = 25). Groups: irradiation control (no BMT; ■); TCD-BM only (●); 5 × 105 Tcons (▾); Tcons + 1 × 104 PBS-57 CD1d− NKT (▵); and Tcons + 1 × 104 PBS-57 CD1d+ NKT (▴). (C) Mice were euthanized at day 39 after transplant. Samples of haired skin, small intestines, large intestines, liver, thymus, spleen, and mesenteric lymph nodes were collected and fixed in formalin for 48 hours before being routinely processed for histologic analysis of stained sections. Evidence for acute GVHD pathology in the haired skin as follows: reduced number of follicles (white arrows), increased inflammation in the panniculus (white asterisk), increased epidermal thickness because of hyperplasia (white measurement bar), increased density of dermal collagen (black asterisk), and mild vacuolar degeneration of the stratum basale with presence of apoptotic bodies (black arrowheads). GVHD-associated colitis was noted with increased numbers of lymphocytes and plasma cells in the lamina propria (black arrows) Increased numbers of intraepithelial lymphocytes are also present. A decreased number of goblet cells is observed, along with evidence of gland regeneration (hyperbasophilia of gland enterocytes, along with increased mitoses and nuclear crowding). Apoptotic bodies (black arrowhead) are present within the glands as well. Severe atrophy (lymphoid depletion) of the lymphoid follicles of splenic white pulp areas (white arrowhead) was noted in the mice receiving Tcon cells only. 100× magnification bar = 200 microns; 200× magnification bar = 100 microns; 600× magnification bar = 25 microns.
Adoptively transferred donor-type NKT cells protect against GVHD. (A) Doses of NKT cells (DX5+TCRβ+CD4+) from C57BL6 donors (H-2b) were adoptively transferred into lethally irradiated BALB/c mice (H-2d) that had TCD-BM and 5.0 × 105 Tcons (to induce GVHD). Significant improvement in survival was observed with the transfer of either 1.0 × 104 or 2.5 × 104 NKT cells (P < .01). Data shown in graph were pooled from 4 independent experiments. Groups: irradiation control (no BMT; ■; n = 25); TCD-BM only (●; n = 25); 5 × 105 Tcons (▾; n = 25); Tcons + 1 × 105 NKT (▵; n = 20); Tcons + 5 × 104 NKT (◇; n = 18); Tcons + 2.5 × 104 NKT (○; n = 23); and Tcons + 1 × 104 NKT (□; n = 19). (B) Further sorting of NKT cells into PBS-57 CD1d–PBS-57 tetramer-positive and tetramer-negative subpopulations indicates that both invariant and noninvariant NKT cells provide protection against GVHD (P < .05). Graph contains data pooled from 4 independent experiments (n = 25). Groups: irradiation control (no BMT; ■); TCD-BM only (●); 5 × 105 Tcons (▾); Tcons + 1 × 104 PBS-57 CD1d− NKT (▵); and Tcons + 1 × 104 PBS-57 CD1d+ NKT (▴). (C) Mice were euthanized at day 39 after transplant. Samples of haired skin, small intestines, large intestines, liver, thymus, spleen, and mesenteric lymph nodes were collected and fixed in formalin for 48 hours before being routinely processed for histologic analysis of stained sections. Evidence for acute GVHD pathology in the haired skin as follows: reduced number of follicles (white arrows), increased inflammation in the panniculus (white asterisk), increased epidermal thickness because of hyperplasia (white measurement bar), increased density of dermal collagen (black asterisk), and mild vacuolar degeneration of the stratum basale with presence of apoptotic bodies (black arrowheads). GVHD-associated colitis was noted with increased numbers of lymphocytes and plasma cells in the lamina propria (black arrows) Increased numbers of intraepithelial lymphocytes are also present. A decreased number of goblet cells is observed, along with evidence of gland regeneration (hyperbasophilia of gland enterocytes, along with increased mitoses and nuclear crowding). Apoptotic bodies (black arrowhead) are present within the glands as well. Severe atrophy (lymphoid depletion) of the lymphoid follicles of splenic white pulp areas (white arrowhead) was noted in the mice receiving Tcon cells only. 100× magnification bar = 200 microns; 200× magnification bar = 100 microns; 600× magnification bar = 25 microns.
Further separation of NKT cells into CD1d–PBS-57 tetramer-positive invariant NKT cells and CD1d–PBS-57 tetramer-negative noninvariant NKT cells indicated that protection against GVHD involved both of these subpopulations (Figure 2B). Transfer of just 1.0 × 104 invariant or noninvariant NKT cells more than doubled the survival rate of mice receiving 5 × 105 Tcons (44% versus 20% survival at day 100, respectively; P = .02 for invariant and P = .04 for noninvariant compared with Tcons alone), indicating that both of these subsets of NKT cells contributed to the suppression of GVHD-associated mortality.
Histologic analysis of formalin-fixed, paraffin-embedded tissues (haired skin, small and large intestines, liver, thymus, spleen, and mesenteric lymph nodes) revealed some significant differences in the occurrence and/or severity of pathology that were consistent with acute GVHD in mice receiving NKT cells and Tcons compared with animals that only received Tcons (Figure 2C). For example, epidermal manifestations of acute GVHD (epidermal hyperplasia, interface dermatitis with apoptotic bodies, dermal fibrosis, dermal inflammation, panniculitis, and follicular inflammation and/or hair loss) were observed in the mice receiving Tcon cells only, but were altogether absent in the mice receiving 2.5 × 104 or 1 × 105 NKT cells. Similarly, whereas no observable lesions were noted in the small intestines or livers of the mice receiving 2.5 × 104 or 1 × 105 NKT cells, lymphocytic enteritis and portal hepatitis were noted in mice receiving Tcons only. All Tcon-only mice also consistently presented with moderate to marked lymphocytic colitis with increased apoptosis in crypt enterocytes; however, these findings were greatly reduced in mice that received 2.5 × 104 or 1 × 105 NKT cells. Interestingly, no observable changes were noted in the thymi or mesenteric lymph nodes in any of the groups, although atrophy of lymphoid follicles in the splenic white pulp areas was consistently less severe in the mice receiving Tcons and 2.5 × 104 or 1 × 105 NKT cells compared with mice receiving Tcons only.
Unlike CD4+CD25+ regulatory cells, NKT cells minimally affect the proliferation of conventional CD4 or CD8 cells at doses that reduce GVHD
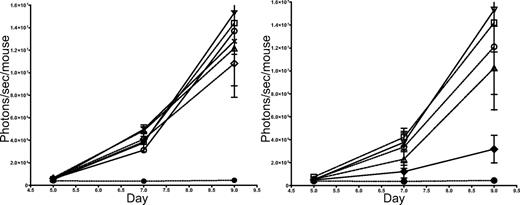
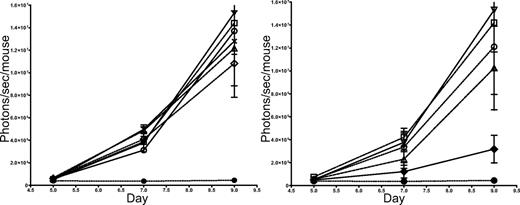
To further examine the mechanism by which NKT cells may decrease GVHD and improve the survival of mice receiving allogeneic Tcons, we examined the effect of NKT cells on the proliferation of Tcons compared with doses of Tregs that suppress GVHD. Using the same allogeneic transplantation model used in Figure 2, we observed by quantitation of the BLI signal from 5 × 105luc+Tcons given on day 2 after transplantation that the addition of 1 × 104, 2.5 × 104, 5 × 104, 1 × 105, or 2.5 × 105 NKT cells did not significantly alter the proliferation of Tcons in vivo (Figure 3 left panel). In marked contrast, as the number of CD4+CD25+ Tregs was increased from 1 × 104 to 2.5 × 105, the in vivo proliferation of luc+Tcons was increasingly suppressed (Figure 3 right panel). We know from previous work with Tregs that these cells result in considerable suppression of Tcon proliferation7-9,26 to reduce the manifestations of GVHD. Thus, GVHD amelioration and improved survival by NKT cells cannot be explained by suppression of proliferation.
NKT cells, in contrast to CD4+CD25+ T regulatory cells, have little effect on Tcon proliferation in vivo. Left: BLI of luc+Tcons in the presence of various quantities of NKT cells shows the expansion of Tcons in vivo to be minimally affected by NKT cells. Groups: TCD-BM only (●); 5 × 105 Tcons (▿); Tcons + 2.5 × 105 NKT (×); Tcons + 1 × 105 NKT (▵); Tcons + 5 × 104 NKT (◇); Tcons + 2.5 × 104 NKT (○); and Tcons + 1 × 104 NKT (□). Bars indicate means ± SE (n = 5). Right: Increasing numbers of CD4+CD25+ Tregs cause a dose-dependent decrease in luc+Tcon proliferation in vivo, as indicated by BLI. Groups: TCD-BM only (●); 5 × 105 Tcons (▿); Tcons + 2.5 × 105 Tregs (♦); Tcons + 1 × 105 Tregs (▵); Tcons + 2.5 × 104 Tregs (○); and Tcons + 1 × 104 Tregs (□). Bars indicate means ± SE (n = 5)
NKT cells, in contrast to CD4+CD25+ T regulatory cells, have little effect on Tcon proliferation in vivo. Left: BLI of luc+Tcons in the presence of various quantities of NKT cells shows the expansion of Tcons in vivo to be minimally affected by NKT cells. Groups: TCD-BM only (●); 5 × 105 Tcons (▿); Tcons + 2.5 × 105 NKT (×); Tcons + 1 × 105 NKT (▵); Tcons + 5 × 104 NKT (◇); Tcons + 2.5 × 104 NKT (○); and Tcons + 1 × 104 NKT (□). Bars indicate means ± SE (n = 5). Right: Increasing numbers of CD4+CD25+ Tregs cause a dose-dependent decrease in luc+Tcon proliferation in vivo, as indicated by BLI. Groups: TCD-BM only (●); 5 × 105 Tcons (▿); Tcons + 2.5 × 105 Tregs (♦); Tcons + 1 × 105 Tregs (▵); Tcons + 2.5 × 104 Tregs (○); and Tcons + 1 × 104 Tregs (□). Bars indicate means ± SE (n = 5)
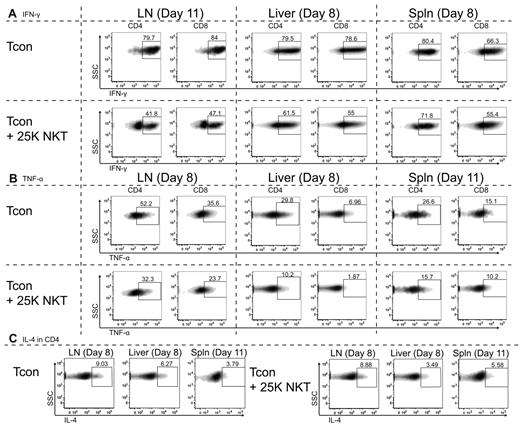
NKT cells lower the percentage of Tcons producing the proinflammatory cytokines IFN-γ and TNF-α
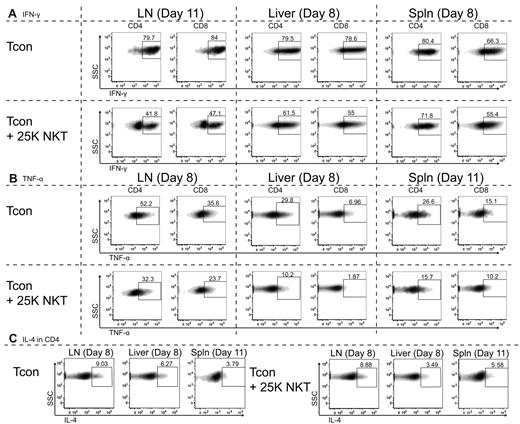
Because we have demonstrated that NKT cells have minimal effects on the proliferation of Tcons, we hypothesized that NKT cells must function through an alternate mechanism. We examined intracellular levels of various cytokines secreted by Tcons in the presence or absence of 2.5 × 104 NKT cells after transplantation. The most significant changes (P < .0001) were observed in the percentages of Tcons expressing the proinflammatory T-helper 1 (Th1) cytokine IFN-γ (Figure 4A). At day 11 after transplant, the presence of 2.5 × 104 NKT cells in the lymph nodes caused a reduction in the IFN-γ–expressing population from 80% and 84% in CD4 and CD8 cells to 42% and 47%, respectively. A decrease in the IFN-γ–positive population was also observed in liver and spleen at day 8, with the NKT cells causing the percentage of IFN-γ–positive CD4 cells to decrease from 80% to 62% and from 80% to 72% in the liver and spleen, respectively. Similarly, the decrease in IFN-γ–positive CD8 cells was also decreased in the presence of NKT cells: from 79% to 55% and from 66% to 55% in the liver and spleen, respectively.
NKT cells affect cytokine production of CD4 and CD8 Tcon. Balb/c recipients (H-2d) were transplanted with TCD-BM and either 5.0 × 105 Tcons or 5.0 × 105 Tcons + 2.5 × 104 NKT cells. Cells were then reisolated at the indicated days after transplantation from lymph nodes, liver, or spleen (spln). FACS analysis of intracellular cytokines was performed with donor-specific and congenic markers to separate NKT cells from Tcons. Shown are representative FACS data showing the median of 3 independent experiments. (A) Cytokine expression of IFN-γ by CD4 or CD8 donor-type Tcons shows the addition of NKT cells to cause a more rapid decline in the percentage of IFN-γ–expressing Tcons. The observed decreases in IFN-γ expression in the presence of NKT cells were very significant (P < .0001) based on an unpaired t test between mean fluorescence intensities of plots shown. (B) Cytokine expression of TNF-α by CD4 or CD8 donor-type Tcons shows that the addition of NKT cells results in a more rapid decline in the percentage of TNF-α–expressing Tcons. The decreases in TNF-α expression in the presence of NKT cells were very significant (P < .0001) based on an unpaired t test between mean fluorescence intensities of plots shown. (C) Cytokine expression of IL-4 by CD4 cells was not significantly altered by the addition of NKT cells, with no apparent trends of increased or decreased expression of IL-4 indicated between experiments.
NKT cells affect cytokine production of CD4 and CD8 Tcon. Balb/c recipients (H-2d) were transplanted with TCD-BM and either 5.0 × 105 Tcons or 5.0 × 105 Tcons + 2.5 × 104 NKT cells. Cells were then reisolated at the indicated days after transplantation from lymph nodes, liver, or spleen (spln). FACS analysis of intracellular cytokines was performed with donor-specific and congenic markers to separate NKT cells from Tcons. Shown are representative FACS data showing the median of 3 independent experiments. (A) Cytokine expression of IFN-γ by CD4 or CD8 donor-type Tcons shows the addition of NKT cells to cause a more rapid decline in the percentage of IFN-γ–expressing Tcons. The observed decreases in IFN-γ expression in the presence of NKT cells were very significant (P < .0001) based on an unpaired t test between mean fluorescence intensities of plots shown. (B) Cytokine expression of TNF-α by CD4 or CD8 donor-type Tcons shows that the addition of NKT cells results in a more rapid decline in the percentage of TNF-α–expressing Tcons. The decreases in TNF-α expression in the presence of NKT cells were very significant (P < .0001) based on an unpaired t test between mean fluorescence intensities of plots shown. (C) Cytokine expression of IL-4 by CD4 cells was not significantly altered by the addition of NKT cells, with no apparent trends of increased or decreased expression of IL-4 indicated between experiments.
Significant changes (P < .0001) were also observed in the percentages of donor Tcons expressing another proinflammatory Th1 cytokine, TNF-α (Figure 4B). At 8 days after transplantation, mice receiving NKT cells had fewer TNF-α–expressing cells in the lymph nodes (CD4: 52% vs 32%; CD8 36% vs 24%). Similar decreases were observed in the liver at day 8 and in the spleen at day 11. The percentage of liver lymphocytes expressing TNF-α was decreased at day 8 from 30% to 10% and from 7% to 2% in CD4 and CD8 cells, respectively. The percentage of splenic donor Tcons expressing TNF-α was also reduced at day 11 from 27% to 16% and from 15% to 10% in CD4 and CD8 cells, respectively.
No significant differences were observed in the cytokine profiles of IL-2–, IL-4–, IL-5–, IL-10–, and IL-17–producing cells (data not shown). Our results indicate that the presence of NKT cells results in an alteration in the cytokine profile of both CD4 and CD8 Tcons, resulting in fewer T cells producing IFN-γ and TNF-α during the course of GVHD.
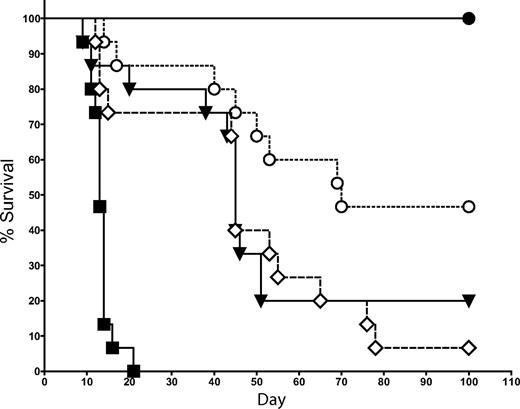
Mechanism of NKT-cell suppression of GVHD involves IL-4 production by NKT cells
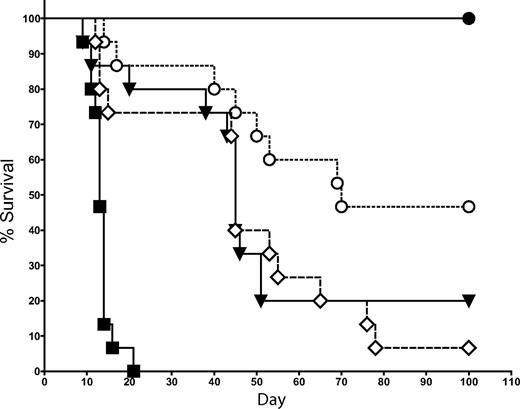
Because IL-4 and IFN-γ are the 2 primary cytokines produced by NKT cells,27-29 we examined whether the suppression of GVHD by NKT cells is dependent on the production of either of these 2 cytokines. In the same transplantation model used in Figure 2, we cotransferred 1.0 × 104 FACS-sorted NKT cells (purity > 95%) from either WT or IL-4−/− KO donor mice (all C57BL/6 background) with 5 × 106 TCD-BM followed by Tcons on day 2. NKT cells from IL-4−/− KO mice were significantly less effective at suppressing GVHD than WT NKT cells (Figure 5; P = .02). No significant decrease in protection against GVHD was observed after the transfer of NKT cells from IFN-γ−/− KO mice compared with WT NKT cells (data not shown). Thus, IL-4 production but not IFN-γ production by NKT cells is an essential component in the suppressive mechanism of NKT cells.
Protection against GVHD depends on IL-4 production by NKT cells. 1.0 × 104 NKT cells (DX5+TCRβ+CD4+) were sorted from C57BL6 donors (H-2b) and transferred into BALB/c mice (H-2d) that had received T cell–depleted bone marrow and 5.0 × 105 Tcons (to induce GVHD). The source of NKT cells was either WT or IL-4−/− KO mice. Data indicated that the transfer of 1.0 × 104 WT NKT cells resulted in significantly improved survival over the transfer of 1.0 × 104 IL-4−/− KO NKT cells (P = .02). Groups: irradiation control (no BMT; ■); TCD-BM only (●); 5 × 105 Tcons (▾); Tcons + 1 × 104 WT NKT (○); and Tcons + 1 × 104 IL-4−/− NKT (◇). Data in graph are pooled from 3 independent transplants (n = 15).
Protection against GVHD depends on IL-4 production by NKT cells. 1.0 × 104 NKT cells (DX5+TCRβ+CD4+) were sorted from C57BL6 donors (H-2b) and transferred into BALB/c mice (H-2d) that had received T cell–depleted bone marrow and 5.0 × 105 Tcons (to induce GVHD). The source of NKT cells was either WT or IL-4−/− KO mice. Data indicated that the transfer of 1.0 × 104 WT NKT cells resulted in significantly improved survival over the transfer of 1.0 × 104 IL-4−/− KO NKT cells (P = .02). Groups: irradiation control (no BMT; ■); TCD-BM only (●); 5 × 105 Tcons (▾); Tcons + 1 × 104 WT NKT (○); and Tcons + 1 × 104 IL-4−/− NKT (◇). Data in graph are pooled from 3 independent transplants (n = 15).
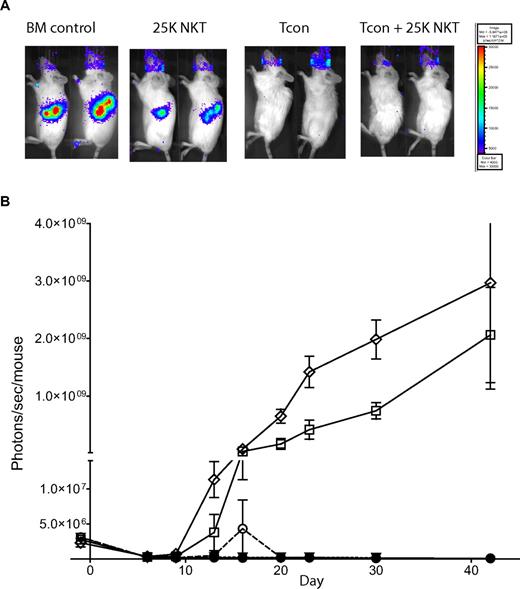
The presence of NKT cells does not interfere with tumor clearance by Tcons
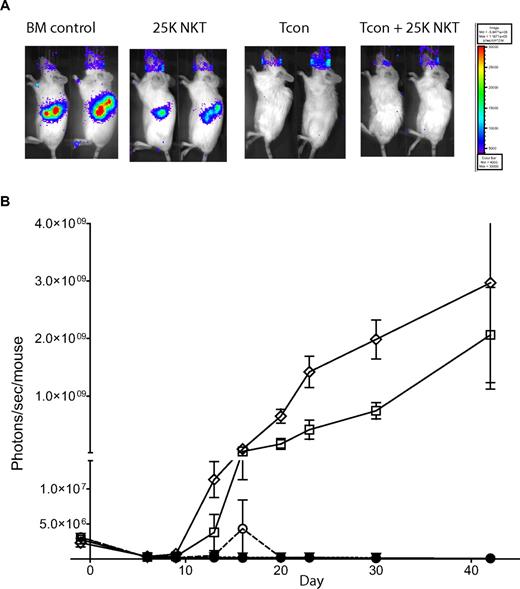
Because HCT is often used to treat hematologic malignancies, we examined whether NKT cells impact the clearance of tumors by Tcons. Using the same allogeneic transplantation model used in Figure 2, we transferred 2.5 × 104 NKT cells alone, 5.0 × 105 Tcons alone, or the 2 populations together into Balb/c recipients that had luc+Bcl-1 lymphoma cells introduced 7 days before transplantation (Figure 6). Mice receiving TCD-BM alone had strong BLI signals from their tumors. The addition of 2.5 × 104 NKT cells alone caused some reduction in tumor burden; however, complete tumor clearance was obtained only when mice received either Tcons or Tcons + NKT cells (Figure 6A). Figure 6B shows that the luc+Bcl-1 tumor burden was initially reduced because of radiation, but then became reestablished by days 7-9, except in the groups receiving either Tcons alone or Tcons + NKT cells (Figure 6B; P < .0001). Interestingly, when mice were treated with Tcons and tumor together, improvement in overall survival was not observed with the addition of NKT cells, because these animals continued to have complete tumor clearance but were more likely to die from GVHD in the long term, suggesting that NKT regulation of GVHD may be attenuated in the presence of tumors (data not shown). These results indicate that the GVT effect is intact in the presence of NKT cells, and that the NKT cells themselves may contribute some modest antitumor activity.
NKT cells do not affect tumor clearance by Tcons. To assess tumor clearance by Tcons, recipient mice were engrafted with luc+Bcl-1 tumor cells 7 days before transplantation. Tumor burden was then monitored via BLI. (A) BLI at day 9 after transplantation of the luc+Bcl-1 lymphoma with TCD-BM alone, Tcons alone, NKT alone, or Tcons + NKT indicated that both groups receiving Tcons were completely cured of their tumors. Shown are 2 representative mice from each group. (B) BLI graph showing tumor load during transplant. Shown is the mean ± SE from one of 2 independent experiments with 5 mice per group. Groups: TCD-BM only (●); Bcl-1 control (◇); Bcl-1 + 5 × 105 Tcons (▿); Bcl-1 + Tcons + 2.5 × 104 NKT (○); and Bcl-1 + 2.5 × 104 NKT (□).
NKT cells do not affect tumor clearance by Tcons. To assess tumor clearance by Tcons, recipient mice were engrafted with luc+Bcl-1 tumor cells 7 days before transplantation. Tumor burden was then monitored via BLI. (A) BLI at day 9 after transplantation of the luc+Bcl-1 lymphoma with TCD-BM alone, Tcons alone, NKT alone, or Tcons + NKT indicated that both groups receiving Tcons were completely cured of their tumors. Shown are 2 representative mice from each group. (B) BLI graph showing tumor load during transplant. Shown is the mean ± SE from one of 2 independent experiments with 5 mice per group. Groups: TCD-BM only (●); Bcl-1 control (◇); Bcl-1 + 5 × 105 Tcons (▿); Bcl-1 + Tcons + 2.5 × 104 NKT (○); and Bcl-1 + 2.5 × 104 NKT (□).
Discussion
In this study, we examined the impact of adoptive transfer of highly purified populations of NKT cells in a well-established murine model of MHC-mismatched HCT. This is the first study to demonstrate the trafficking and survival of NKT cells after adoptive transfer in an allogeneic transplantation model. We observed a robust expansion of NKT cells in secondary lymphoid organs, followed by migration to GVHD target organs in a pattern similar to that of Tcons.25 NKT cells had remarkably long survival; however, unlike CD4 and CD8 Tcons that induce lethal acute GVHD at similar cell doses, did not cause any significant mortality.
We observed that the transfer of very low numbers of donor NKT cells suppressed GVHD, improved survival past day 100, and involved both invariant and noninvariant NKT cells. Interestingly, the dose of NKT cells that was 1/20 of the Tcon dose was found to be the most protective, and increasing the dose of NKT cells to 1/10 or 1/5 of the Tcon dose resulted in less improvement in survival. These differences are also reflected in the pathology of the skin and gastrointestinal tract, in that mice receiving Tcons alone had moderate to severe lymphocytic colitis, increased crypt enterocytes, apoptotic bodies, and other manifestations of acute GVHD. Mice receiving a low dose of NKT cells had relatively healthy-appearing gastrointestinal tracts with almost no signs of GVHD. Mice receiving a high dose of NKT cells, however, had pathology between that of the low dose of NKT cells and the Tcon-only group. Together, the survival and pathology data suggest that NKT cells may have a yet to be determined “side effect” that is detrimental to the mice when NKT cells are given at higher doses. However, we have shown that mice can receive 5.5 × 105 NKT cells alone without any increased morbidity or mortality; therefore, if high doses of NKT cells cause adverse effects, then these effects are dependent on the presence of GVHD-inducing Tcons. The reasons that the higher doses of NKT cells were less effective at suppressing GVHD are likely to be complex and are beyond the scope of this study. However, we can speculate that because NKT cells are potent producers of IFN-γ,19 they might contribute to Tcon-mediated gastrointestinal pathology at high doses.
Interestingly, even though small numbers of NKT cells were capable of suppressing GVHD, they had little effect on Tcon proliferation. This finding is in marked contrast to the adoptive transfer of CD4+CD25+ Tregs, which also suppress GVHD yet function through the suppression of Tcon proliferation, as has been observed in this and previous studies.7,8 CD4+CD25+ Tregs suppress both Tcon proliferation and consequently GVHD with more potency as their numbers are increased. Therefore, we concluded that NKT cells must ameliorate GVHD via a different mechanism. Furthermore, NKT cells had an optimal dose of just 1-2.5 × 104 cells, which is considerably less than has been observed in previous studies with Tregs, which typically require 5 × 105 cells for biologic activity in this same strain combination.7-9
Production of the proinflammatory Th1 cytokines IFN-γ and TNF-α by Tcons are important in GVHD pathophysiology. Reports from both murine and human studies indicate that massive cytokine release related to the Th1 phenotype of alloreactive T cells predicts the incidence and severity of acute GVHD.30 In addition, Th1/Th2 polarization of T-helper cell subsets affects the development of GVHD, determining end organ damage.31 After myeloablative conditioning and transfer of Tcons, several changes occur in the gastrointestinal tract. Initially, there is a proliferation that results in crypt lengthening and increased intraepithelial lymphocytes linked to IFN-γ production.32 IFN-γ increases MHC class II expression and gut permeability33 and possibly affects crypt stem-cell turnover.34 Inhibiting IFN-γ reduces gut pathology in animals with GVHD.35 IFN-γ production by Tcons also primes monocytes and macrophages, making them sensitive to lipopolysaccharides in the gut36 (released by radiation and T cell–induced damage). These cells then produce TNF-α that is directly toxic to the gut during GVHD.37-39 Therefore, a variety of strategies have been explored to alter the proinflammatory function of donor Tcons that have affected GVHD severity.26,35,39-41 NKT cells significantly and consistently down-regulated the production of proinflammatory IFN-γ and TNF-α by Tcons; therefore, it is not surprising that addition of NKT cells results in healthier gastrointestinal tracts and improved survival.
This decrease in the Th1 cytokines IFN-γ and TNF-α in the donor Tcon population suggested that the effect of NKT cells may be attributed to their ability to produce IL-4. This appears to be the case. NKT cells from IL-4−/− KO mice were significantly less effective in suppressing GVHD than were WT NKT cells. This result is consistent with other studies that have also found IL-4 to be a mechanism by which NKT cells may suppress GVHD.11,16 Furthermore, stimulation of recipient mice with the NKT ligand α-GalCer has also been shown to reduce GVHD in a mechanism dependent on IL-4 production by recipient NKT cells.42 In addition, several groups have found that adoptive transfer of IL-4–producing, Th2-polarized cells can reduce GVHD.43-47 Because the mechanism of NKT-cell suppression of GVHD is dependent on IL-4, we expected to see more Th2-type, IL-4–expressing T cells in the presence of NKT; however, at this early point in GVHD induction we have primarily observed high levels of IFN-γ and TNF-α, and most T cells present in the mouse are not naive. Therefore, it would likely take more time for Th0 progenitors to be induced toward a Th2, IL-4 producing phenotype if this were to occur. Nevertheless, we believe that the decrease in Th1 cytokine–producing cells (including the inhibition of the formation of new Th1 cells), is sufficient to decrease GVHD without an increase in the Th2 population. In contrast to the findings by Kim et al16 that IFN-γ–producing bone marrow type II NKT cells suppressed GVHD by inducing the apoptosis of donor T cells, we did not find IFN-γ−/− KO NKT cells to be significantly less effective than WT NKT cells in suppressing GVHD in our model.
Because HCT is commonly used as therapy for hematologic malignancies, we assessed whether NKT-cell regulation of Tcons would affect their GVT effect. We found that the addition of NKT cells alone caused some reduction in tumor burden, in contrast to Tregs, which do not have any significant antitumor activity7 ; however, complete tumor clearance was obtained only when mice received both Tcons and NKT cells. These results indicate that the GVT effect is intact in the presence of NKT cells, and that the NKT cells themselves may provide some antitumor activity. This is consistent with numerous reports in the literature that NKT cells, particularly invariant NKT cells, have antitumor activity.48 However, we did not find survival to be improved by NKT cells in the presence of both Tcons and tumor, because in the presence of tumor the ability of NKT cells to protect against GVHD was reduced.
In conclusion, we have shown the proliferation and migration of CD4+NKT cells in a model of allogeneic bone marrow transplantation, and found that low doses of NKT cells ameliorate GVHD pathology and improve animal survival. In contrast to CD4+CD25+ Tregs, NKT cells do not reduce GVHD by suppressing the proliferation of Tcons, but rather act via a mechanism involving IL-4 production and cause a decrease in Th1-type cytokine production by donor T cells. Furthermore, the addition of NKT cells did not interfere with the GVT effect of conventional T cells. We have also elucidated potential caveats regarding the use of NKT cells in that they were less effective at higher doses and their regulatory function may be attenuated in the presence of tumor. These data support the potential therapeutic application of allogeneic NKT cells in clinical allogeneic HCT, but also indicate a need for continued research into the behavior of NKT cells and their interactions with their environment and other immune cells to gain further insight into immune modulation of GVHD and T-cell function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all members of the Negrin laboratory for helpful discussions and technical assistance. PBS-57–loaded mCD1d tetramers were provided by the National Institutes of Health Tetramer Facility.
This work was supported by grants P01 HL075462 and P01 CA049605 from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
Authorship
Contribution: D.B.L.-G. designed and performed research, analyzed data, and wrote the manuscript; J.A.O., E.I.S., J.B., and R.Z. performed research; R.H.L. performed research, helped analyze data, and helped write the manuscript; and R.S.N. designed experiments, provided overall guidance, and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Science Research Building Rm 2205, 269 W Campus Dr, Stanford University, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
J.A.O. and E.I.S. contributed equally to this study.