Abstract
We examined the role that N-linked glycans play in the synthesis and expression of von Willebrand Factor (VWF). Blocking the addition of N-linked glycans (NLGs) or inhibiting initial glycan processing prevented secretion of VWF. To determine whether specific glycosylation sites were important, the 16 VWF N-linked glycosylation sites were mutated followed by expression in HEK293T cells. Four NLG mutants affected VWF expression: N99Q (D1 domain), N857Q (D' domain), N2400Q (B1 domain), and N2790Q (CK domain) either abolished or reduced secretion of VWF and this was confirmed by metabolic labeling. Multimer analysis of mutant N2790Q cell lysate revealed an increase in VWF monomers, which was also observed when the isolated CK domain was expressed with N2790 mutated. Immunofluorescence microscopy showed that mutants N99Q, N857Q, and N2790Q were primarily retained within the ER, producing only few pseudo Weibel-Palade bodies over longer time periods compared with wtVWF. All the variants also showed an increase in free thiol reactivity. This was greatest with N857Q and D4-C2 NLG mutants, which had approximately 6-fold and 3- to 4-fold more free thiol reactivity than wtVWF. These data provide further evidence of the critical role that individual N-linked glycans play in determining VWF synthesis and expression.
Introduction
von Willebrand factor (VWF) is a large multimeric plasma glycoprotein essential for normal hemostasis, acting firstly by supporting platelet adhesion to surfaces at sites of vascular injury and secondly as the carrier molecule for pro-coagulant Factor VIII (FVIII).1,2 Synthesis of VWF is limited to megakaryocytes and endothelial cells.3 The pre–pro-VWF molecule comprises a 22 amino acid signal peptide, a 741 amino acid propeptide, and a 2050 amino acid mature subunit. The pro-VWF monomer is composed of 4 types of domains (A-D) arranged as follows: NH2-D1-D2-D'-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK-COOH.1 VWF multimers are formed by C- and N-terminal intermolecular disulphide bonds,4,5 with the largest multimers exceeding 2 × 104 kDa and having the greatest adhesive activity.6 During synthesis, VWF undergoes extensive posttranslational modification resulting in the addition of 12 N-linked and 10 O-linked glycosylation sites per mature monomer.7 Furthermore, the propeptide also contains 4 potential N-linked glycosylation sites although it is not known if these are used. In total, carbohydrate accounts for approximately 20% of the molecular weight of VWF.8
N-linked glycosylation is one of the most common co/posttranslational modifications. It is characterized by the addition of a carbohydrate moiety to the protein via a beta-glycosidic linkage between an N-acetylglucosamine residue and the δ-amide of an asparagine residue in the sequence NXS/T (or in some cases NXC),9 where X is any amino acid except proline.10 This takes place after translocation into the endoplasmic reticulum (ER) when the enzyme oligosaccharyltransferase (OST) transfers a preformed 14-saccharide core unit (Glc3Man9GlcNAc2) en bloc onto the nascent peptide chain.11 Glucosidases I and II rapidly remove the 2 terminal glucose residues from this unit and the resulting monoglucosylated Glc1Man9 GlcNAc2 structure becomes a ligand for the resident ER lectin chaperones calreticulin (CRT) and calnexin (CNX).12-14 During the association with CRT or CNX, a complex is formed with ERp57, a member of the protein disulphide isomerase family which acts as an oxidoreductase, assisting the formation and isomerization of disulphide bonds.15 When the protein is correctly folded, the final glucose residue is removed by glucosidase II, allowing release from CRT or CNX and signaling that the protein is ready to continue its transit through the ER to the Golgi. Misfolded or unassembled proteins can be reglucosylated by UDP-glucose:glycoprotein glucosyltransferase (UGT) and then reassociate with CRT or CNX.15 After removal of the final glucose, ER and Golgi mannosidases further process the carbohydrate structure to a Man3GalNAc2 form and then in the medial and trans-Golgi, glycosyltransferases add GlcNac and galactose sugars to produce ‘complex’ glycans chains.11 These may be capped by the addition of sialic acid to produce the fully processed N-linked glycan chain. It is notable that in endothelial cells, where VWF is synthesized, the fucosyl transferase FUT1 competes with sialyl transferases to add fucose to the end of the chain, thus creating the H-antigen of blood group O which can be further modified by the addition of a GalNAc or galactose residue if the subject is of blood group A or B, respectively.16
N-linked glycans play a variety of important roles in the production of functional proteins including facilitation of protein folding, disulphide bond formation, prevention of aggregation, stabilization and protection from proteolysis.17,18 However, although NLG sites are often highly conserved, the precise function of individual NLG at specific sites, both within and outside the cell is often unclear. In a previous study we demonstrated that N-linked glycosylation of VWF modulated the interaction with the VWF cleaving protease ADAMTS13 and one glycosylation site in particular, N1574, was a major determinant of ADAMTS13 binding affinity and subsequent cleavage of VWF.19 In the present study we have investigated further the functional significance of individual N-linked glycans on VWF and demonstrate that occupancy of selective N-linked glycosylation sites are critical for the proper intracellular processing of VWF, affecting rates of dimer and intramolecular disulphide bond formation and playing crucial roles in mediating secretion and storage of VWF.
Methods
VWF expression vectors
The expression vector pcDNA-FL-VWF has been previously described.19 The QuikChange XL site directed mutagenesis kit (Stratagene) was used to generate 16 point mutations at sites N99, N156, N211, N666, N857, N1147, N1231, N1515, N1574, N2223, N2290, N2357, N2400, N2546, N2585, and N2790, in each case changing the asparagine for glutamine (Figure 1A). All mutations were verified by DNA sequencing to ensure the presence of the correct mutation and the absence of any other randomly introduced mutations. Additional mutations, T101A, T859A, T2402A and S2792A, were prepared in the same way. A construct expressing the isolated CK domain, termed VWF-CK (residues 2720-2813), was also created. In brief, polymerase chain reaction (PCR) was used to amplify the CK domain of VWF followed by a stop codon and an AgeI restriction site and a PCR method20 was used to insert this fragment (with or without the mutation encoding N2790Q) into a modified pcDNA3.1 vector that contained the VWF signal peptide followed by an N-terminal Myc/His tag.
Lectin analysis of the VWF propeptide
The presence of glycans in the VWF propeptide (VWFpp) was determined using a modified lectin enzyme-linked immunosorbent assay (ELISA). MaxiSorp plates (Nunc) were coated overnight with 5 μg/mL a monoclonal anti-VWFpp antibody, CLB-Pro35 (Sanquin reagents; Mast Group LTD). After washing and blocking with 2.5% powdered milk in Tris buffered saline with 0.1% Tween-20 (TBS-T), serial dilutions of normal pooled plasma or rVWF were added to the wells and incubated at room temperature for 2 hours. Bound protein was detected with either anti-VWFpp antibody CLB-Pro 14.3 (Sanquin reagents) conjugated to HRP, or a polyclonal goat anti-VWF antibody directed against epitopes in the CK domain (Santa Cruz Biotechnology; clone sc8068). Specific biotinylated lectins (Vector Laboratories), Concanavalin A, Elderberry bark lectin, and Ulex europeaus, diluted in TBS supplemented with 1mM CaCl2, 1mM MgCl2 and 2mM MgCl2 pH 7.4, were used to assess sugar composition. Bound lectins were detected with strepavidin-HRP (Dako) diluted 1/10 000. Bound antibody was measured with Sigma color fast OPD substrate (Poole).
Expression and analysis of recombinant VWF
FL-VWF, its variants, and the VWF-CK domain constructs were transiently expressed in either HEK293T or HEK293 cells in serum free media, using 1 μg/mL DNA and 10mM PEI, as previously described.19 Transfections were performed in 6 well plates and media and lysates were harvested 3 days after transfection. Cells were lysed in CHAPS lysis buffer with protease inhibitors (Sigma-Aldrich; 0.5% CHAPS, 150mM NaCl, 1mM CaCl2, 20mM HEPES, pH 8.0) for 30 minutes on ice and then centrifuged for 10 minutes at 8000g to pellet cell debris. To analyze expression in the presence of N-linked glycosylation inhibitors cells were placed in Optimem 4 hours before transfection in the presence or absence of either 1 μg/mL tunicamycin, 100 μg/mL castanospermine and 10 μg/mL swainsonine (all from Sigma-Aldrich). At 12 hours after transfection, the media was further supplemented with the glycosylation inhibitors at the stated concentrations. In some assays Lactacystin (Merck) was added to the cells during transfection at a final concentration of 10μM. The analysis of VWF by ELISA, reducing and nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and VWF multimer gel analysis were all performed as previously described.19 VWF-CK domain was detected with mouse monoclonal anti-myc antibody (Santa Cruz Biotechnology) and goat anti–mouse HRP (Dako).
Pulse-chase analysis of VWF expression
Metabolic labeling was performed essentially as described.21 Transfections were performed as described in the previous paragraph and cells were incubated for 3 days. Cells were then washed with warm PBS and incubated with Methionine and Cysteine free DMEM (Invitrogen) for 60 minutes and then pulse labeled with 400μCi EasyTag EXPRESS35S Protein Labeling Mix (PerkinElmer[b]) for 60 minutes. Cells were then washed once with warm phosphate buffered saline (PBS), reincubated with Optimem, and chased for stated time periods. Lysates were prepared using CHAPS lysis buffer and all samples were precleared with Protein G Sepharose for 60 minutes at room temperature. Immunoprecipitation was performed using Dynabeads Protein G (Invitrogen). Protein G Dynabeads, 40 μL, were incubated with 5 μg of polyclonal rabbit anti-VWF (Dako) for 15 minutes then washed with PBS supplemented with 0.1% Tween-20, and then added to media or lysate samples and incubated at room temperature for 15 minutes with up and over mixing. Immunoprecipitates were washed once with CHAPS lysis buffer and twice with PBS-T then eluted with 2 X Lithium dodecyl sulfate reducing buffer for 20 minutes at 95°C and electrophoresed through 4% to 12% Bis-Tris gels (Invitrogen). Gels were fixed with propanol:water:acetic acid (25:65:10) for 30 minutes and then incubated with 20 mL of Amplify reagent for 30 minutes then dried and exposed to MP Hyperfilm (both GE Healthcare).
Immunofluorescence microscopy
To identify the intracellular location of VWF, HEK293 cells were seeded on to glass coverslips placed in the wells of 12 well tissue culture plates (Nunc) and were transfected with 1 μg/mL DNA using PEI and incubated for 5 days. Glass coverslips were removed and rinsed once with warm phosphate buffered saline (PBS), fixed and permeabilized using ice-cold methanol for 5 minutes at −20°C and air dried for 20 minutes. Coverslips were stained with rabbit polyclonal anti-VWF (Dako) diluted 1/1000 in PBS supplemented with 2% BSA for 45 minutes in the dark at room temperature. After 5 washes with PBS, coverslips were incubated with goat anti–rabbit AlexaFluor 488 (Invitrogen) for 15 minutes in the dark at room temperature. Coverslips were then washed 10 times with PBS and mounted onto microscope slides using Vectorshield (Vector Laboratories) and visualized under a laser scanning confocal microscope LSM510 META (Carl Zeiss) using a Plan Apochromat 63×/1.4 objective lens. Images were analyzed using LSM imaging software (Carl Zeiss) and processed using Adobe Photoshop CS2. To determine the number of cells forming Webiel Palade bodies (WPB), 10 separate fields of view were taken for each variant from 4 separate transfections and the number of cells with at least 5 visible WPB was counted. Data were expressed as the percentage of the total cells with 5 or more visible WPBs.
Analysis of free thiol content of VWF
The free thiol content of VWF was assessed essentially as previously described.22 Equal concentrations (0.5 μg/mL) of wild type (wt) or mutant VWF or normal pooled plasma diluted in PBS were incubated with 100μM malemide-PEO2-Biotin (MPB; Thermo) for 10 minutes at room temperature and the reaction quenched with 200 μM GSH. Samples were then diluted with PBS-T to final concentration 0.25 μg/mL and applied to MaxiSorp plates that had been previously coated with polyclonal anti-VWF antibodies and incubated for 1 hour at room temperature. MPB was detected by incubation with Strepavadin-HRP (Dako) diluted 1/1000 in PBS-T and parallel samples were detected with anti–VWF-HRP to ensure equal concentrations of VWF were being analyzed. Bound antibody was measured with Sigma color fast OPD as before. Some samples treated with 5mM N-Ethylmaleimide (NEM) for 30 minutes before incubation with MPB as a control. A FL-VWF mutant containing an additional free thiol, Y1584C, was used as a positive control. The amount of MPB bound was expressed as a percentage by taking the absorbance at 492 nm for bound Streptavadin-HRP / absorbance 492nM bound anti–VWF-HRP ×100.
Results
N-linked glycosylation and initial N-linked glycan modification is required for efficient cellular processing and secretion of VWF
The role of N-linked glycosylation in synthesis and secretion of VWF was initially examined by expressing VWF in the presence of inhibitors of different stages of N-linked glycan processing and analyzing expression levels by VWF ELISA (Figure 1B). In keeping with previous reports, expression of VWF in the presence of tunicamycin, which blocks the attachment of the precursor N-linked glycan chain to the nascent peptide, abolished secretion of VWF.23 The amount of VWF in the cell lysates was also reduced suggesting that the nonglycosylated VWF was targeted for intracellular degradation. Castanospermine is a glucosidase inhibitor that prevents initial glucose trimming and has been shown to inhibit glycan dependent interaction with the ER lectin chaperones CNX and CRT.24 When VWF was expressed in the presence of castanospermine, secretion of VWF was virtually abolished, while the amount retained in the cell lysates was similar to wtVWF, suggesting that interaction with calnexin and calreticulin is required to assist in the processing of VWF (Figure 1B). VWF was also expressed in the presence of swainsonine to inhibit the conversion of high mannose to complex glycans. This had no effect on VWF secretion, indicating that further processing of VWF N-linked glycan chains in the Golgi is not essential for VWF secretion (Figure 1B). Multimer gel analysis showed that VWF expressed in the presence of tunicamycin was observed only in the lysate as a monomer, suggesting that glycosylation is essential for dimer formation (Figure 1C). In contrast, the CST treated cell lysates showed VWF that was able to form dimers and a few tetramers, implying that interaction with lectin chaperones may not be required for dimer formation (Figure 1C). The multimeric pattern of VWF expressed in the presence of SW was similar to wtVWF.
Effect of N-linked glycosylation inhibitors on VWF expression and glycosylation of the VWFpp. (A) Domain diagram of von Willebrand Factor (VWF) showing the location of the 16 N-linked glycosylation sites, 4 in the propeptide and 12 in each mature subunit. (B) Wild-type (wt) VWF was transiently expressed in HEK293T in the presence of 1 μg/mL tunicamycin (TM), 100 μg/mL castanospermine (CST), or 10 μg/mL swainsonine (SW) and VWF levels in the cell media (■) or lysate (□) analyzed by ELISA 3 days after transfection. Error bars represent mean ± SEM of 3 separate experiments each performed in duplicate. (C) The multimer composition of VWF expressed with glycosylation inhibitors was determined by electrophoresis in 1.4% agarose gels followed by Western blotting. (D) Microtitre plates coated with anti–VWF-propeptide CLB-Pro35 were used to capture plasma (■) or recombinant (□) VWFpp and the glycan content assessed using lectins Concanavalin A (Con A), Elderberry bark lectin (EBL), and Ulex europeaus (Ulex. E). Anti–VWF-propeptide-HRP (CLB-Pro 14.3) was used to confirm capture of the propeptide and an anti–VWF-CK domain antibody was used to confirm the absence of mature VWF.
Effect of N-linked glycosylation inhibitors on VWF expression and glycosylation of the VWFpp. (A) Domain diagram of von Willebrand Factor (VWF) showing the location of the 16 N-linked glycosylation sites, 4 in the propeptide and 12 in each mature subunit. (B) Wild-type (wt) VWF was transiently expressed in HEK293T in the presence of 1 μg/mL tunicamycin (TM), 100 μg/mL castanospermine (CST), or 10 μg/mL swainsonine (SW) and VWF levels in the cell media (■) or lysate (□) analyzed by ELISA 3 days after transfection. Error bars represent mean ± SEM of 3 separate experiments each performed in duplicate. (C) The multimer composition of VWF expressed with glycosylation inhibitors was determined by electrophoresis in 1.4% agarose gels followed by Western blotting. (D) Microtitre plates coated with anti–VWF-propeptide CLB-Pro35 were used to capture plasma (■) or recombinant (□) VWFpp and the glycan content assessed using lectins Concanavalin A (Con A), Elderberry bark lectin (EBL), and Ulex europeaus (Ulex. E). Anti–VWF-propeptide-HRP (CLB-Pro 14.3) was used to confirm capture of the propeptide and an anti–VWF-CK domain antibody was used to confirm the absence of mature VWF.
The VWF propeptide contains complex N-linked glycans but not the H-antigen
While 12 NLG sites have been described in the mature VWF monomer it is not known if the 4 potential NLG sites in the VWF propeptide (VWFpp) are occupied by N-linked glycan chains. We used the N-linked glycosylation prediction server NetNGlyc (available from http://www.cbs.dtu.dk/services/NetNGlyc) to predict the likelihood of these sites being used. The NetNGlyc server returns an N-linked glycosylation potential value of between 0 and 1 for each site with a value of more than 0.5 indicating that the sites is likely to be occupied. All 4 sites in the VWFpp; N99, N156, N211, and N666 returned values of more than 0.5 and are therefore likely to be glycosylated. We performed lectin binding analysis of the propeptide using a modified VWFpp ELISA, as shown in Figure 1D. Both VWFpp in pooled plasma and recombinant wtVWF bound Con A, demonstrating the presence of N-linked glycans. Both plasma and recombinant VWFpp also bound EBL, indicating the presence of sialic acid and confirming the presence of complex glycan structures. In keeping with previous observations they did not bind to Ulex europaeus, indicating the absence of H-antigen.25 The specificity of the assay for VWFpp was confirmed by the ability of the samples to bind anti-VWFpp, but not an antibody directed against the C terminal region of VWF (Figure 1D).
Specific N-linked glycan mutations affect expression and secretion of VWF
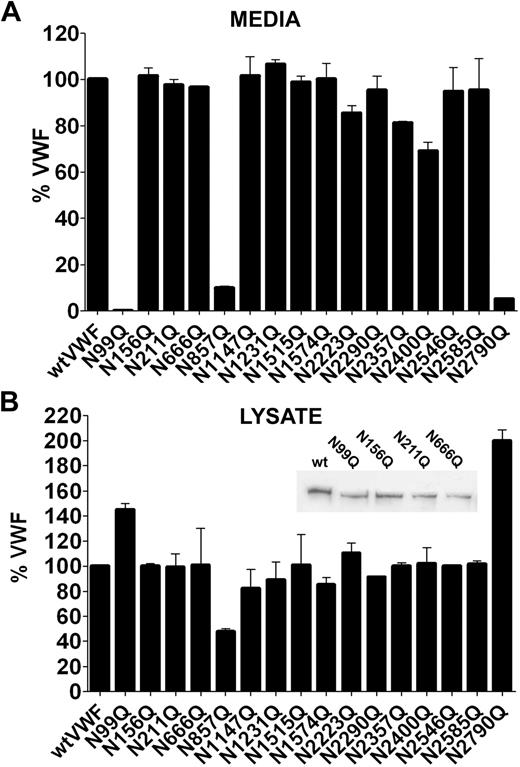
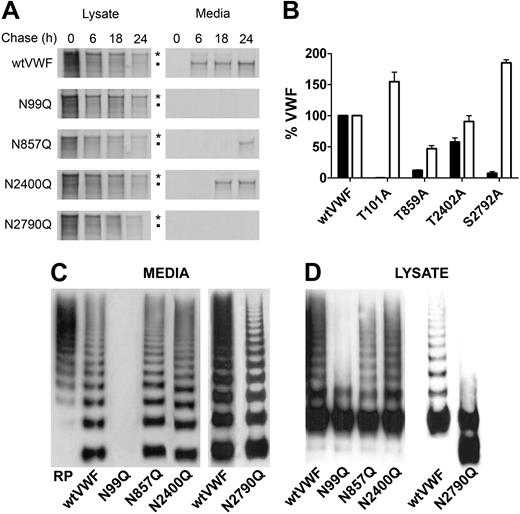
We then expressed VWF with mutations of each of the 16 sites present in the molecule. Interestingly, only 4 mutations affected VWF secretion; N99Q in the propeptide, N857Q, N2400Q, and N2790Q in the mature subunit (Figure 2A). Mutation of N99 virtually abolished secretion of VWF, with the majority of the protein retained within the cell lysate (Figure 2B), indicating that the first N-linked glycosylation event in the ER after translocation is essential for VWF processing. Mutation of N857 (Figure 2A) reduced secretion by approximately 90% compared with wtVWF. Reduced mutant VWF levels were also observed in the cell lysate compared with wtVWF. Mutation of N2400, located in the B2 domain, reduced secretion by approximately 30% (Figure 2A), but similar VWF levels to wtVWF were seen the cell lysate (Figure 2B). Mutation of N2790, located in the CK domain, showed minimal secretion (Figure 2A) and greatly increased levels in the cell lysate (Figure 2B), indicating intracellular retention but without accelerated degradation. To confirm if all 4 sites in the VWFpp were occupied by an N-linked glycan chain we analyzed cell lysate samples in 3% to 8% SDS-PAGE gels under reducing conditions. Compared with wtVWF each of the 4 VWFpp glycan mutants migrated slightly further through the gel consistent with the loss of a glycan chain (Figure 2B inset). To confirm the expression data, we performed pulse-chase experiments to provide a more detailed analysis of VWF secretion (Figure 3A). Transfected HEK293 cells were pulse labeled with 35S-Cys-Met and chased for 6, 18, and 24 hours. In accordance with previous reports, radiolabeled wtVWF appeared in the media after 6 hours as both pro- and mature-VWF. By 18 hours the only band visible in the media corresponded to mature VWF and this was accompanied by a reduction of wtVWF in the cell lysate. Compared with wtVWF, little or no secretion was observed for mutations N99Q, N857Q, and N2790Q, with labeled VWF being retained in the cell lysate. Secretion of VWF-N2400Q was observed but to a lesser extent than wtVWF, confirming the expression studies. Interestingly, the disappearance of VWF-N857Q from the cell lysate appeared markedly increased compared with wtVWF, suggesting rapid degradation within the cell. To determine whether the observed effects on expression of VWF-N99Q, N857Q, N2400Q, and N2790Q were due to loss of the glycan chain and not the amino acid mutation we constructed further mutants by mutating the third amino acid in the glycosylation consensus sequence N-X-S/T to alanine, thereby preserving the asparagine but preventing its glycosylation. Expression of VWF-T101A, T859A, T2402, and S2792A in HEK293T cells demonstrated similar expression profiles to the asparagine mutations, with absence or reduction of secretion of all variants and reduction of mutant T895A in the cell lysate (Figure 3B). This suggests that it is the loss of the glycan chain that is responsible for the phenotype observed.
Effect of N-linked glycosylation mutation on the expression and secretion of VWF. VWF N-linked glycan mutants were expressed in HEK293T cells and VWF concentrations in the secreted media (A) and cell lysate (B) were analyzed by VWF ELISA. Error bars represent mean ± SEM of 3 separate experiments each performed in duplicate. Inset: Cell lysate samples of wtVWF and the VWFpp glycosylation mutants were analyzed by reducing SDS-PAGE in 3% to 8% gels followed by Western blotting with polyclonal anti–VWF-HRP
Effect of N-linked glycosylation mutation on the expression and secretion of VWF. VWF N-linked glycan mutants were expressed in HEK293T cells and VWF concentrations in the secreted media (A) and cell lysate (B) were analyzed by VWF ELISA. Error bars represent mean ± SEM of 3 separate experiments each performed in duplicate. Inset: Cell lysate samples of wtVWF and the VWFpp glycosylation mutants were analyzed by reducing SDS-PAGE in 3% to 8% gels followed by Western blotting with polyclonal anti–VWF-HRP
Further analysis of the expression of VWF N-linked glycan mutants. (A) Conditioned media and cell lysate samples were collected after 0, 6, 18, and 24 hours chase and immunoprecipitated with Dynabeads protein G and polyclonal anti-VWF antibodies. Immunoprecipitates were analyzed by SDS-PAGE in 4% to 12% gels under reducing conditions and autoradiography. *: Pro-VWF; ■: mature VWF. (B) Glycosylation at N99, N857, N2400, and N2790 was blocked by mutating the third amino acid in the N-linked glycosylation consensus sequence. Mutants were expressed in HEK293T cells and expression analyzed by VWF ELISA. Error bars represent mean ± SD of 3 separate experiments each performed in duplicate. (C-D) The multimeric structure of wtVWF, N99Q, N857Q, N2400Q, and N2790Q in both the expressed media (C) and cell lysate (D) was assessed in 1.4% agarose gels and visualized with HRP-conjugated anti-VWF polyclonal antibodies.
Further analysis of the expression of VWF N-linked glycan mutants. (A) Conditioned media and cell lysate samples were collected after 0, 6, 18, and 24 hours chase and immunoprecipitated with Dynabeads protein G and polyclonal anti-VWF antibodies. Immunoprecipitates were analyzed by SDS-PAGE in 4% to 12% gels under reducing conditions and autoradiography. *: Pro-VWF; ■: mature VWF. (B) Glycosylation at N99, N857, N2400, and N2790 was blocked by mutating the third amino acid in the N-linked glycosylation consensus sequence. Mutants were expressed in HEK293T cells and expression analyzed by VWF ELISA. Error bars represent mean ± SD of 3 separate experiments each performed in duplicate. (C-D) The multimeric structure of wtVWF, N99Q, N857Q, N2400Q, and N2790Q in both the expressed media (C) and cell lysate (D) was assessed in 1.4% agarose gels and visualized with HRP-conjugated anti-VWF polyclonal antibodies.
Next, we analyzed the multimeric pattern of the N-linked glycan mutants from both media and lysate samples. All the mutants that were expressed normally had a multimeric content similar to that of wtVWF (data not shown). To analyze the secreted VWF-N857Q and N2790Q mutants we concentrated the media approximately 15-fold before loading onto the gel. Interestingly, although both VWF-N857Q and N2790Q had significantly reduced secretion, the multimeric composition of the protein was comparable to wtVWF (Figure 3C), indicating that the protein that does transit the ER undergoes normal multimerization. Analysis of the cell lysates showed mainly the presence of a strong dimer band with faint bands of higher order multimers (Figure 3D). Compared with wtVWF the lysate of VWF-N99Q only showed dimers and a few tetramers (Figure 3D). The mutation of N99 therefore most likely results in ER retention of the molecule. Significantly, the lysate of VWF-N2790Q showed mainly dimers and monomeric VWF (Figure 3D), indicating that loss of the glycan in the CK domain affects dimer formation in the ER.
Glycosylation mutants VWF-N99Q, N857Q, and N2790Q are retained within the ER and reduce VWF storage
Because 3 of the NLG mutants, VWF-N99Q, N857Q, and N2790Q, affected VWF secretion and were retained within the cell, we investigated the intracellular fate of these mutants. Expression of VWF in HEK293 cells has previously been shown to induce the formation of storage vesicles indistinguishable from WP bodies and these cells were therefore used for this investigation.26
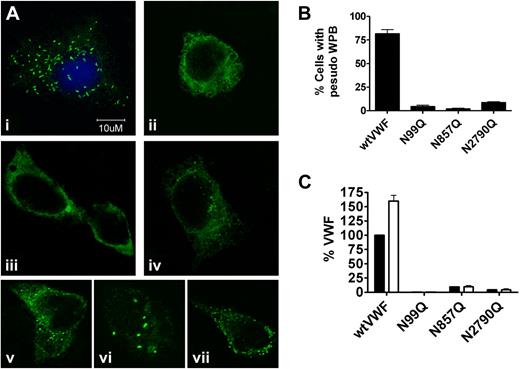
Five days after transfection, HEK293 cells were fixed and permeabilized, stained for VWF then analyzed by immunofluorescence confocal microscopy (Figure 4). Consistent with previous observations, cells transfected with wtVWF demonstrated 3 distinct staining patterns: (1) diffuse granular staining that colocalized with the ER marker calnexin (data not shown), (2) diffuse granular staining with a number of elongated or rounded punctate structures that have previously been shown to be pseudo-WPBs, and (3) little or no diffuse staining with just the presence of pseudo WPB.26 After 5 days transfection the majority of wtVWF transfected cells demonstrated little granular staining and numerous WPBs indicating correct processing and storage of VWF (Figure 4Ai). For mutants VWF-N99Q, N857Q, and N2790Q, most of the cells demonstrated diffuse granular ER, staining confirming that these mutants impaired normal processing of the VWF molecule resulting in ER retention (Figures 4Aii-iv) Interestingly though, for all 3 mutants a small number of cells were able to form VWF-containing inclusion bodies (Figures 4Av-vii). It is not clear that these are the same as WPB and the number of cells in which they formed was very much less than wtVWF; approximately 3% for the N99Q and N857Q mutants and 9% for the N2790Q mutant compared with approximately 76% for wtVWF (Figure 4B). To determine whether these possible WPBs and their VWF could be stimulated normally, we treated cells with PMA 5 days after transfection to stimulate WPB release. After PMA stimulation, an increase in wtVWF in the secreted media was observed. In contrast, PMA treatment had no effect on the secretion of the VWF-N99Q, N857Q, and N2790Q mutants (Figure 4C). This either reflects the inability of these WPB to release VWF which may be incorrectly folded or may simply reflect the small number of cells able to form WPBs.
Intracelluar localization of VWF N-linked glycan mutants. Five days after transfection, HEK293 cells were fixed and stained with polyclonal rabbit anti-VWF and goat anti–rabbit AlexaFluor 488 and visualized under a laser scanning confocal microscope LSM510 META. Images in panels Ai through iv are representative images of typical staining patterns observed for (i) wtVWF, (ii) N99Q, (iii) N857Q, and (iv) N2790Q. Images in panels Av through vii are representative of a small number of cells transfected with N99Q (v), N857Q (vi), and N2790Q (vii) that were able to form pseudo Webiel-Palade bodies. (B) Ten separate fields of view were taken for each variant from 4 separate experiments and the number of cells with at least 5 visible WPB counted and expressed as a percentage of the total number of cells expression WPB. (C) Five transfection HEK293 cells were stimulated with 100 ng/mL PMA and VWF concentration analyzed by VWF ELISA. ■: No PMA; □, plus PMA
Intracelluar localization of VWF N-linked glycan mutants. Five days after transfection, HEK293 cells were fixed and stained with polyclonal rabbit anti-VWF and goat anti–rabbit AlexaFluor 488 and visualized under a laser scanning confocal microscope LSM510 META. Images in panels Ai through iv are representative images of typical staining patterns observed for (i) wtVWF, (ii) N99Q, (iii) N857Q, and (iv) N2790Q. Images in panels Av through vii are representative of a small number of cells transfected with N99Q (v), N857Q (vi), and N2790Q (vii) that were able to form pseudo Webiel-Palade bodies. (B) Ten separate fields of view were taken for each variant from 4 separate experiments and the number of cells with at least 5 visible WPB counted and expressed as a percentage of the total number of cells expression WPB. (C) Five transfection HEK293 cells were stimulated with 100 ng/mL PMA and VWF concentration analyzed by VWF ELISA. ■: No PMA; □, plus PMA
Loss of glycosylation at N857 results in proteasomal degradation
Because the expression of the VWF-N857Q mutant reduced secretion and the amount of VWF in the cell lysate, we expressed this mutation in the presence of the proteasomal inhibitor lactacystin that has previously been shown to delay intracellular degradation of VWF with the von Willebrand disease causing mutation C1149R.21 Expression of wtVWF in the presence of lactacystin marginally reduced secretion and increased intracellular retention of VWF (Figure 5A). However, expression of VWF-N857Q with lactacystin increased the amount of VWF protein in the lysate to a level comparable to wtVWF, suggesting that mutation at this site causes accelerated degradation by the proteasome. Pulse chase analysis of the lysate from cells transfected with N857Q in the presence or absence of lactacystin also confirmed this observation, with lactacystin delaying removal from the lysate (Figure 5B).
Loss of glycosylation at N857 causes intracellular degradation by the proteasome. (A) Wild-type VWF and VWF-N857Q were expressed in the absence or presence of 10μM Lactacystin and expression analyzed by VWF ELISA Secreted media (■) and cell lysate (□). (B) HEK293T cells transfected with VWF-N857Q and subjected to pulse chase analysis in the absence or presence of 10μM Lactacystin. Cell lysates were immunoprecipitated and analyzed as described in “Methods.” *: Pro-VWF, ■: mature VWF.
Loss of glycosylation at N857 causes intracellular degradation by the proteasome. (A) Wild-type VWF and VWF-N857Q were expressed in the absence or presence of 10μM Lactacystin and expression analyzed by VWF ELISA Secreted media (■) and cell lysate (□). (B) HEK293T cells transfected with VWF-N857Q and subjected to pulse chase analysis in the absence or presence of 10μM Lactacystin. Cell lysates were immunoprecipitated and analyzed as described in “Methods.” *: Pro-VWF, ■: mature VWF.
N-linked glycosylation of the CK domain at N2790 is required for efficient dimer formation
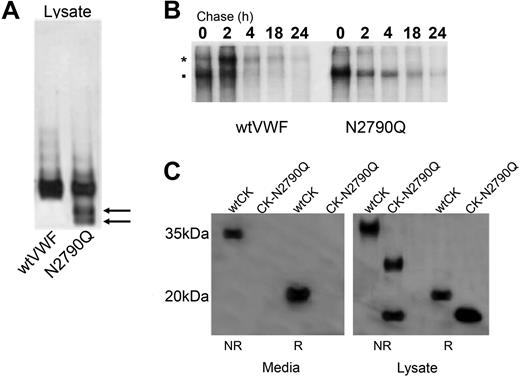
Multimer analysis of the cell lysate of VWF-N2790Q shown in Figure 3 demonstrated the presence of VWF monomers that are not normally abundant. We repeated multimer analysis of the VWF-N2790Q lysates with reduced protein concentrations and noted that the monomeric band was composed of 2 separate bands corresponding to pro-VWF and the mature VWF subunit (Figure 6A). Pulse-chase analysis and nonreducing SDS-PAGE of cell lysates after transfection with wtVWF showed increasing amounts of dimers with a small number of monomers, whereas VWF-N2790Q lysate showed increasing amounts of monomers with very little conversion to dimeric VWF (Figure 6B). To determine whether the effect of N2790Q was localized to the CK domain, we prepared a construct encoding the CK domain with an N-terminal His-Tag. The N2790Q mutation was introduced into this vector and expressed in HEK293T cells. Under nonreducing SDS-PAGE, wtVWF-CK-domain was present both in the secreted media and the cell lysate as a dimer. VWF-CK-N2790Q was not secreted and under nonreducing conditions was present in the cell lysate as both faster migrating dimers and monomers consistent with the absence of the N-linked glycan chain and confirming the observation seen with full-length VWF that glycosylation of N2790 is required for efficient dimer formation (Figure 6C).
N-linked glycosylation of the VWF CK domain. (A) Multimer analysis was performed on lysate samples from HEK293T cells transfected with wtVWF and VWF-N2790Q. Mutant N2790Q media was concentrated approximately 20-fold for multimer analysis. (B) Cell lysate samples were collected at chase times 0, 2, 4, 18, and 24 hours and after immunoprecipitation were analyzed by SDS-PAGE under nonreducing conditions. *: Pro-VWF, ■: mature VWF. (C) The VWF CK domain wild type and N2790Q were transiently expressed in HEK293T cells and conditioned media and cell lysate samples analyzed by SDS-PAGE under reducing and nonreducing conditions. CK domain was detected with mouse monoclonal anti-myc antibody followed by goat anti–mouse HRP. The difference in migration between wtCK and CK-N2790Q confirms the absence of the N-linked glycan chain.
N-linked glycosylation of the VWF CK domain. (A) Multimer analysis was performed on lysate samples from HEK293T cells transfected with wtVWF and VWF-N2790Q. Mutant N2790Q media was concentrated approximately 20-fold for multimer analysis. (B) Cell lysate samples were collected at chase times 0, 2, 4, 18, and 24 hours and after immunoprecipitation were analyzed by SDS-PAGE under nonreducing conditions. *: Pro-VWF, ■: mature VWF. (C) The VWF CK domain wild type and N2790Q were transiently expressed in HEK293T cells and conditioned media and cell lysate samples analyzed by SDS-PAGE under reducing and nonreducing conditions. CK domain was detected with mouse monoclonal anti-myc antibody followed by goat anti–mouse HRP. The difference in migration between wtCK and CK-N2790Q confirms the absence of the N-linked glycan chain.
Mutation of VWF N-linked glycan sites affects disulphide bond formation and free thiol content
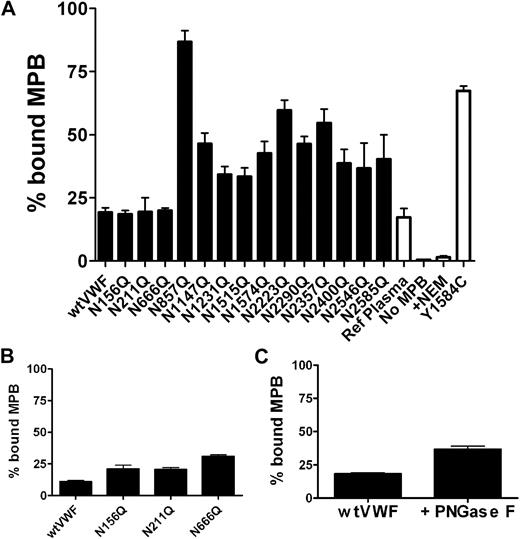
Because the N2790Q mutant affected dimer formation we hypothesized that the other N-linked glycan sites could also be involved in mediating disulphide bond formation. Indeed, previous studies have demonstrated that N-linked glycosylation favours pairing of cysteine residues by lowering the amount of free energy required for disulphide bond formation.27,28 VWF is a highly cysteine rich molecule and we noted that many of the N-linked glycosylation sites are in close proximity to several cysteine residues and may therefore assist efficient disulphide bond formation. Equal amounts of wt and mutant VWF were incubated with Maleimide-PEO2-biotin to react with free thiols and the reaction quenched with GSH. A modified VWF ELISA was then performed to determine the amount of bound MPB. As a control and calibrant we expressed the Y1584C variant of VWF that has an extra unpaired cysteine residue per monomer. Consistent with more recent reports, evidence of unpaired cysteine residues was obtained with purified plasma derived VWF,29 (Figure 7A). Recombinant wtVWF also reacted similarly with MPB, demonstrating the presence of free thiols. When VWF was prior treated with 20mM NEM, no MPB binding was observed. Interestingly with the exception of the VWFpp mutants, all the other N-linked glycan mutants (except N2790Q that could not be analyzed due to its low expression level) showed an increased reaction with MPB, indicating an increase in the presence of free thiols. However, the effect was largest with VWF-N857Q which presented approximately 5-fold more bound MPB than wtVWF (Figure 7A). Unexpectedly, the VWF-N1515Q and N1574Q mutants also showed increased MPB binding, despite these 2 glycosylation sites not being located in the immediate vicinity of any cysteine residues. To investigate the effect of the glycan mutations on the VWFpp mutants, the MPB binding assay was repeated, except the VWFpp was captured by the anti-VWFpp antibody. Interestingly, the recombinant wtVWFpp also bound MPB indicating the presence of unpaired cysteines and MPB binding was increased with the VWFpp mutants (Figure 7B). Loss of glycans within the propeptide did not affect the free thiol content of the mature VWF molecule.
Effect of VWF N-linked glycans of free thiol content. (A) VWF at 0.5 μg/mL was incubated with 100μM MPB before quenching with 200μM GSH. Samples were captured onto microtitre plates with polyclonal anti-VWF antibodies. Bound VWF was detected with anti–VWF-HRP and bound MPB with streptavidin-HRP. (B) The free thiol content of the recombinant VWFpp was determined in the same way, except labeled VWFpp was captured with anti-VWFpp antibody. (C) Wild-type VWF was treated with PNGase F overnight at 37°C and then labeled with MPB and captured to microtitre plates with polyclonal anti-VWF antibody. Error bars represent mean ± SEM of 3 separate experiments each performed in triplicate.
Effect of VWF N-linked glycans of free thiol content. (A) VWF at 0.5 μg/mL was incubated with 100μM MPB before quenching with 200μM GSH. Samples were captured onto microtitre plates with polyclonal anti-VWF antibodies. Bound VWF was detected with anti–VWF-HRP and bound MPB with streptavidin-HRP. (B) The free thiol content of the recombinant VWFpp was determined in the same way, except labeled VWFpp was captured with anti-VWFpp antibody. (C) Wild-type VWF was treated with PNGase F overnight at 37°C and then labeled with MPB and captured to microtitre plates with polyclonal anti-VWF antibody. Error bars represent mean ± SEM of 3 separate experiments each performed in triplicate.
To investigate whether the increased MPB binding we observed with the N-linked glycan mutants was due to increased exposure of free thiols arising from alteration of the protein conformation, we analyzed the free thiol content of PNGase F treated wtVWF. Previously, we have shown that removal of VWF N-linked glycan chains by PNGase F results in a more open configuration of the VWF molecule.19 Interestingly PNG-VWF also demonstrated a modest increase in bound MPB (Figure 7C). This was comparable to the increase seen with several of the single mutants but somewhat less than the increase we observed with the VWF-N857Q, N1147Q, N1231Q, N2223Q, and N2357Q mutants.
Discussion
In the present study we have investigated the role that N-linked glycosylation plays in the synthesis and secretion of VWF. In accord with previous observations, blocking the addition of N-linked glycans with tunicamycin abolished secretion of VWF,23 while blocking the conversion from high mannose to complex structures with swainsonine had no effect on either expression or multimerization. Significantly, Castanospermine treatment also abolished VWF secretion suggesting that the interaction of VWF with the lectin like chaperones CNX and/or CRT is required for effective VWF processing.
To establish whether overall glycosylation or specific N-linked sites were important for different aspects of VWF synthesis and secretion, we constructed point mutations removing each of the 16 N-linked glycan sites via a conservative Asn to Gln substitution to minimize disruption of the polypeptide chain. Only 3 of the NLG sites were found to be essential for normal VWF synthesis and secretion, N99, N857, and N2790. Mutation of a fourth site, N2400, had a smaller effect on VWF secretion. Similar results were obtained when we abolished the N-linked glycosylation site by mutating the serine or threonine residue to alanine in the N-X-S/T sequence at sites T101, T859, and S2792, confirming that the secretion defects were due to the loss of the N-linked glycan chain.
Confocal microscopy confirmed that the VWF-N99Q, N857Q, and N2790Q mutants were all predominantly retained within the ER, although a small proportion of transfected cells were able to form WPB. The small numbers of pseudo WPB formed with these mutations may reflect a stochastic phenomenon in which a number of molecules are by chance assembled normally into WPB or that transient transfection overloads the cellular regulatory mechanisms allowing mis-folded proteins to aggregate in inclusion bodies.30
Mutation of N99 abolished secretion of VWF with the protein being retained within the ER. In preliminary analyses, we demonstrated for the first time, using lectin analysis, that the VWF propeptide contained complex N-linked glycans. In keeping with a previous report, no H-antigen was detected on VWFpp purified from plasma.25 The effect of the N99 mutation suggests that the initial glycosylation event is crucial for efficient processing. N-linked glycosylation occurs cotranslationally as the nascent peptide chain is translocated into the ER and it has been demonstrated that the location of the initial N-linked glycan site determines which chaperone proteins the protein interacts with. If the first glycan site is within 50 amino acids, interaction is normally with CNX/CRT. However if the glycan is after the first 50 amino acids, the protein normally interacts with BiP first, before further interaction with CNX and CRT.31 Prevention of glycosylation at N99 may therefore alter the ability of VWF to interact with the correct chaperone pathway and result in mis-folding of the newly forming peptide.
Mutation N857Q decreased secretion of VWF and also the amount observed in the cell lysate. Expression of VWF-N857Q in the presence of lactacystine restored normal levels in the lysate, indicating that rapid intracellular degradation of this mutant occurs. Combined with the observation that VWF-N857Q had an increased amount of free thiol, this suggests that glycosylation at this site is essential for mediating the correct architecture of the multiple disulphide bonds present in the D'D3 region of VWF. This could be due to the presence of the glycan chain directly facilitating disulphide bond formation, or by mediating interaction with ER PDIs such as ERp57 that have been shown to be involved in VWF synthesis.32
Glycosylation of the CK domain at N2790 was shown to be required for efficient dimer formation, with an intracellular accumulation of VWF monomers resulting from the N2790Q mutant. This explains the previous observation that the dimerization of VWF is blocked by tunicamycin.23 It is also consistent with the role of glycosylation of the CK domains in Muc2 and human chorionic gonadotrophin which similarly affect dimer formation.33,34 It is not known precisely how glycosylation promotes dimer formation but in the case of the Muc2, dimers were shown to form at an increased rate, but to be less stable and to rapidly revert to monomers.33 It has been demonstrated that core glycan residues can interact with neighbouring amino acids and decrease flexibility of the local peptide region, stabilizing the protein structure.35 The lack of effect produced by VWF expressed with swainsonine demonstrates that the full complex glycan structure is not necessary for these putative functions. The glycan at N2790 may therefore stabilize the conformation of the CK domain and favor efficient dimer formation. Alternatively, the presence of the glycan chain may modulate interactions with chaperones to facilitate dimer formation.
Despite the demonstrated importance of N-linked glycosylation for VWF synthesis, there are no clear examples of naturally occurring mutations causing VWD mediated by a direct effect on glycosylation. Only 2 mutations directly affecting an N-linked glycan site are recorded on the VWF database. The mutation N1231T has been reported several times in patients with VWD but appears to represent a gene conversion event and is not considered to be causative for VWD.36 Similarly, although N2546Y was reported in association with type 3 VWD, there was an absence of mRNA containing this mutation, suggesting that another mutation was present in cis which completely prevented expression.37 Interestingly, a recent study demonstrated a novel VWD causing mutation, N528S, which predicts the generation of a new glycosylation site at N526 in the VWFpp. The phenotype of this mutation was defective multimerization, storage and secretion. The introduction of a new glycosylation site could potentially affect the interaction of the propeptide with mature VWF or disrupt the nearby CGLC disulphide isomerase sequence, that is implicated in VWF multimerization38
As well as the specific effect on disulphide bond formation in the CK domain, all the glycan mutants presented an overall increased free thiol reactivity compared with wtVWF. Removal of N-linked glycan chains may therefore lead to a more open VWF conformation allowing exposure and detection of previously buried cysteine residues. Indeed, PNGase F treatment of VWF increased the measured free thiol content, suggesting that some free cysteines are buried in globular VWF. However, for the majority of the mutants the increase in free thiol produced by the loss of a single glycan was greater than that produced by the removal of all N-linked glycans with PNGase F and with the exception of N1515 and N1574, all the remaining sites occur in close proximity to cysteine residues. It is therefore likely that the N-linked glycans of VWF directly modulate the intracellular formation of disulphide bonds and also maintain the globular structure of the molecule preventing exposure of buried unpaired cysteine residues. Although VWF-N1515Q and N1574Q mutants have no nearby cysteine residues, the recently published crystal structure of the VWF A2 domain revealed that the adjacent cysteine residues at the C-terminus end of the A2 domain are paired and N1515 and N1574 might conceivably play a role in modulating disulphide bond formation.39
In summary, our results provide further evidence of the critical role that individual N-linked glycans play in determining VWF synthesis, expression and function. Remarkably, only 3 of the 16 N-linked glycosylation sites in VWF proved to be essential for synthesis and secretion. We postulate that N99 is essential because, as the first N-linked glycan site, it directs the nascent polypeptide to the appropriate chaperones. N2790 appears to be essential for dimerization, without which the monomers cannot leave the ER. Finally, N857 is within a highly folded domain with numerous disulphide bonds and loss of this glycan seems to result in abnormal folding targeting the molecule for degradation. Intriguingly, the VWF N-linked glycan sites are all highly conserved across species, suggesting an unknown function for the remaining 13 sites.
Presented, in part, in abstract form at the XXII Congress of the International Society on Thrombosis and Haemostasis, Boston, MA, July 19, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the British Heart Foundation (PG/06/111 and RG/06/007).
National Institutes of Health
Authorship
Contribution: T.A.J.M. and M.A.L. designed the study, performed experiments, analysed results, and wrote the manuscript; E.C.G., A.N.N., and A.C.K.C performed experiments; G.M.B. designed part of the study, performed experiments, analysed results, and wrote the manuscript; and D.A.L. analysed results and wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Thomas McKinnon, Department of Haematology, Imperial College London, 5th Fl, Commonwealth Bldg, Hammersmith Hospital Campus, Du Cane Rd, London W12 0NN, UK; e-mail t.mckinnon03@imperial.ac.uk.