In this issue of Blood, Dietrich and colleagues report disappointing results with the use of melphalan parenterally with dexamethasone in patients with immunoglobulin light chain amyloidosis. The median survival of 61 patients was 17.5 months. The 3 months all cause mortality was 28%.1
The first demonstration that survival was prolonged in amyloidosis with the use of melphalan-based therapy was in the context of a phase 3 randomized trial.2 In that study, the median survival achieved was only 18 months. Subsequently, the amyloidosis center in Pavia, Italy, reported on 46 patients with light chain amyloidosis ineligible for stem cell transplantation, treated with melphalan and dexamethasone and demonstrated a progression-free survival of 3.8 years and median overall survival of 5.1 years.3 This regimen is now widely considered to be the standard for patients who do not receive stem cell transplants.
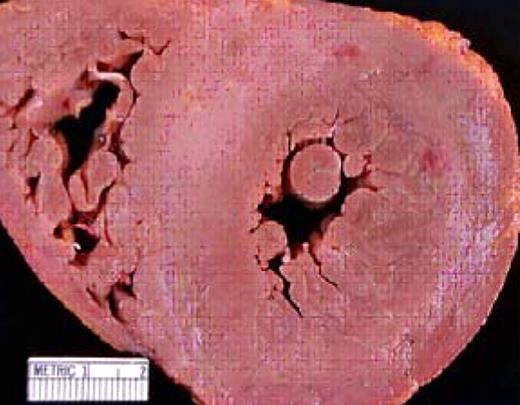
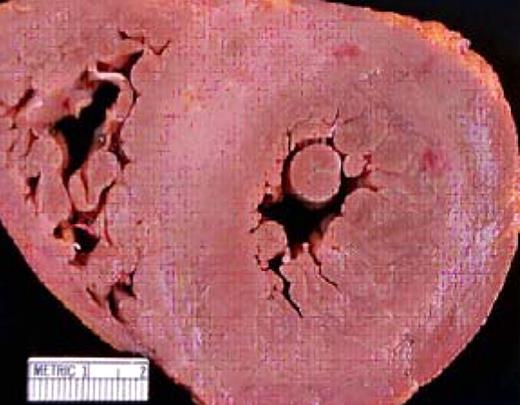
An autopsy specimen that demonstrates cardiac amyloid. The concentric streaks of whitish material in the myocardium represent the “lardaceous change” reported by Von Rokitansky in 1842. Note the dramatic thickening of the interventricular septum (normal is 10 mm).
An autopsy specimen that demonstrates cardiac amyloid. The concentric streaks of whitish material in the myocardium represent the “lardaceous change” reported by Von Rokitansky in 1842. Note the dramatic thickening of the interventricular septum (normal is 10 mm).
How is it possible for a change from melphalan and prednisone to melphalan and dexamethasone to triple the response rate and survival? There appear to be 2 factors that help account for at least some of this difference. The first is that the assessment of response using the immunoglobulin-free light chain assay did not exist in the early studies of melphalan-based therapy but has been used regularly since 2005, resulting in improved ability to monitor hematologic responses.4
Second, the mix of patients is very important in determining overall outcomes. Using a similar melphalan-and-dexamethasone–based regimen, physicians at Weill-Cornell School of Medicine reported a median survival of only 10.5 months.5 The failure of stem cell transplantation to demonstrate a survival benefit in a phase 3 trial compared with melphalan and dexamethasone is in part related to the fact that the median survival in the high-dose melphalan group was only 22.2 months. This poor outcome was in part related to the 28% treatment-related mortality in the high-dose therapy group.6
Is it possible for large populations of patients with light chain amyloidosis to be so heterogeneous from center to center with resultant disparate outcomes? A comparison of 2 large amyloidosis centers in Europe and in the United States covering the 10-year period that predates the introduction of novel agents and stem cell transplantation reported median survivals of 30 months versus 12 months. The group with the shorter survival was older and had a significantly higher proportion of patients with cardiac amyloidosis. Age, performance status, cardiac involvement, and the number of organs involved all independently predicted survival.7 Thus, comparison of outcomes across phase 2 studies is fraught with risks of misinterpretation of data. Outcomes appear to be strongly linked to the proportion of patients with cardiac amyloidosis (see figure).
The major predictor of survival in light chain amyloidosis is the extent of cardiac functional compromise and can be estimated by measurement of cardiac troponin-T and NT-proBNP (N terminal pro Brain naturetic peptide). Patients who have significant elevations of both cardiac biomarkers have a median survival of only 3.5 months. If only 1 cardiac biomarker is significantly elevated, the median survival is only 10.5 months. Inclusion of a high proportion of these patients in any study of treatment will skew the results to shortened survival, which also would make it difficult to demonstrate the value of new therapeutic agents in less advanced disease.8
It has been suggested that patients with stage III light chain amyloidosis be excluded from trials of therapy or be reported separately. In a trial of lenalidomide with or without dexamethasone, almost half of the patients enrolled in the study (10 of 23) had to discontinue treatment within the first 3 cycles of therapy due to 4 early deaths, 3 adverse events, and 3 other causes. The level of cardiac biomarkers accurately predicted which patients were most likely not to complete 3 cycles of therapy.9
Clearly with these poor outcomes, there is a need for improved therapy. Recently, bortezomib has been reported to have activity in light chain amyloidosis.10 However, this agent has not been extensively tested in patients with class III or class IV New York Heart Association cardiac failure.11
What are the take-home messages? First, therapy for the treatment of light chain amyloidosis remains a challenge. Those patients who need treatment most benefit the least. Second, it is dangerous to make treatment-based decisions simply on the outcomes of single-institution phase 2 trials because patient selection plays as much a role in outcome as the specifics of therapy. Third, the introduction of the free light chain level helps facilitate assessment of responses following treatment and the introduction of cardiac biomarkers will help stratify future studies so their comparability is improved. As noted in the study by Dietrich et al, only 9% of their patients were stage I amyloidosis while 53% were stage III, which likely accounts for the poor reported outcomes. Although melphalan and dexamethasone is the current standard of therapy, its ability to provide benefit is highly dependent on selection of the correct patients. A multinational study comparing melphalan and dexamethasone to melphalan, dexamethasone, and bortezomib is scheduled to begin this year. Enrollment in studies of treatment remains the only way to advance our knowledge in this field where improvement in outcomes remains a major therapeutic challenge.
Conflict-of-interest disclosure: M.A.G. has received honoraria from Celgene & Millennium. ■