Tumor relapse after human leukocyte antigen–matched allogeneic stem cell transplantation (SCT) remains a serious problem, despite the long-term presence of minor histocompatibility antigen (MiHA)–specific memory T cells. Dendritic cell (DC)–based vaccination boosting MiHA-specific T-cell immunity is an appealing strategy to prevent or counteract tumor recurrence, but improvement is necessary to increase the clinical benefit. Here, we investigated whether knockdown of programmed death ligand 1 (PD-L1) and PD-L2 on monocyte-derived DCs results in improved T-cell activation. Electroporation of single siRNA sequences into immature DCs resulted in efficient, specific, and long-lasting knockdown of PD-L1 and PD-L2 expression. PD-L knockdown DCs strongly augmented interferon-γ and interleukin-2 production by stimulated T cells in an allogeneic mixed lymphocyte reaction, whereas no effect was observed on T-cell proliferation. Moreover, we demonstrated that PD-L gene silencing, especially combined PD-L1 and PD-L2 knockdown, resulted in improved proliferation and cytokine production of keyhole limpet hemocyanin–specific CD4+ T cells. Most importantly, PD-L knockdown DCs showed superior potential to expand MiHA-specific CD8+ effector and memory T cells from leukemia patients early after donor lymphocyte infusion and later during relapse. These data demonstrate that PD-L siRNA electroporated DCs are highly effective in enhancing T-cell proliferation and cytokine production, and are therefore attractive cells for improving the efficacy of DC vaccines in cancer patients.
Introduction
Alloreactive CD8+ T cells play a crucial role in graft-versus-tumor (GVT) responses after allogeneic stem cell transplantation (alloSCT) and donor lymphocyte infusion (DLI).1,2 In human leukocyte antigen (HLA)–matched alloSCT, these alloreactive CD8+ T-cell responses are directed against minor histocompatibility antigens (MiHAs), which are HLA-bound peptides derived from polymorphic genes that differ between donor and recipient.2,3 Upon infusion into the preconditioned patient, donor-derived MiHA-specific CD8+ T cells clonally expand and become effector cells, which subsequently have the capacity to target MiHA-expressing malignant cells.4 After the primary immune response, most MiHA-reactive CD8+ T cells die through apoptosis, and only a small pool of long-lived memory cells that is able to respond quickly upon reencounter of the antigen survives.5 Importantly, we and others have shown that after DLI, the emergence of MiHA-specific CD8+ effector T cells coincides with a decrease in the number of malignant cells.6,–8 Moreover, years after the initial response, MiHA-specific CD8+ memory T cells can still be detected, playing an important role in protective immunity in transplanted patients. However, these alloreactive CD8+ memory T cells do not always respond efficiently to recurring tumor cells and this failure may contribute to the occurrence of tumor relapses.6 This lack of responsiveness may result from inefficient memory T-cell activation due to absence of effective antigen presentation, impaired costimulation and/or enhanced signaling by coinhibitory receptors. Interestingly, we recently found that the programmed death (PD)–1/PD–ligand (PD-L) axis is involved in inhibiting the function of MiHA-specific CD8+ T cells upon their engagement with PD-L1 expressing myeloid leukemia cells and dendritic cells (DCs; Norde et al, manuscript in preparation).
PD-1 is a coinhibitory receptor that is inducibly expressed by T and B cells upon activation. Engagement of this receptor with either of its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273), results in phosphorylation of the cytoplasmic tail and negatively regulates T-cell receptor (TCR)–induced cytokine production and proliferation via the phosphatidylinositol-3 kinase pathway.9,10 Whereas PD-L2 expression is mainly restricted to activated lymphocytes and antigen-presenting cells (APCs), such as DCs and macrophages, PD-L1 is also expressed by many nonlymphoid tissues and tumor cells.9,11 In the past few years, a crucial role for the PD-1/PD-L1 pathway has been demonstrated in chronic viral infections, such as HIV and hepatitis C virus (HCV).12,,–15 It has been shown that HIV- and HCV-specific T cells have increased PD-1 expression, resulting in functional exhaustion of these cells. Moreover, PD-1/PD-L interactions have been implicated in the functional impairment of tumor antigen–reactive CD8+ T cells in solid tumors and chronic myeloid leukemia.16,–18 Therefore, tumor cells may exploit the same pathway to escape eradication by MiHA-specific CD8+ memory T cells after alloSCT.
DC vaccination therapy would be an appealing strategy to revive functionally inactive MiHA-specific CD8+ memory T cells and, by this means, restore an effective immune response against recurring or persistent MiHA-expressing tumor cells. DCs have been widely exploited as adjuvants in vaccination therapy because of their capability to effectively initiate and reactivate T cell–based immune responses. In GVT and graft-versus-host disease (GVHD) responses, the process of alloreactive T-cell stimulation and activation is also orchestrated by MiHA-presenting DCs.19 Currently, the most commonly used cell source for DC generation is peripheral blood monocytes, because high numbers of monocytes can be easily obtained and differentiated into mature DCs (mDCs).20,21 Using interleukin (IL)-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF), immature DCs (iDCs) are obtained after 5-7 days of culture, followed by 2 days of maturation in the presence of a cytokine cocktail containing IL-1β, IL-6, tumor necrosis factor α (TNF-α), and prostaglandin E2 (PGE2). Upon maturation, the expression of peptide-HLA complexes is increased, and many accessory molecules involved in cell adhesion, cell migration, and costimulation are acquired. For instance, CCR7 plays an important role in DC migration to draining lymph nodes, whereas adhesion molecules CD54 and CD58 are involved in T-cell activation.22 Furthermore, mature DCs have a high expression of costimulatory molecules CD80, CD86, and inducible T-cell costimulator ligand (ICOS-L). However, coinhibitory molecules, such as PD-L1, PD-L2, herpes virus entry mediator (HVEM), and B7-H3, are also highly up-regulated. Expression levels and interactions of these co-stimulatory and -inhibitory ligands, with their counterreceptors on T cells, ultimately determine the turn of the balance toward antigen (Ag)–specific T-cell activation or inhibition.22,–24
Here, we investigated whether the knockdown of PD-1 ligands in monocyte-derived DCs results in improved T-cell function. We identified small-interfering RNAs (siRNAs) that efficiently silenced PD-L1 and PD-L2 expression after electroporation of iDCs. We demonstrated that PD-L gene silencing resulted in significant improvement of interferon (IFN)-γ and IL-2 production, and proliferation of keyhole limpet hemocyanin (KLH)-experienced T cells. Moreover, PD-L knockdown DCs showed superior potential to expand PD-1–expressing MiHA-specific CD8+ effector and memory T cells from leukemia patients shortly after DLI and, later, during relapse. Together, these findings indicate that PD-L–silenced DCs are attractive cells for improving the efficacy of DC vaccines in cancer patients.
Methods
Patient and donor material
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donor buffy coats (Sanquin Blood Bank). Blood samples were collected after written informed consent was obtained in ongoing clinical SCT and DC vaccination protocols, approved by the Radboud University Nijmegen Medical Center (RUNMC) Institutional Review Boards. PBMC containing KLH-specific T cells were obtained from multiple myeloma (MM) patients vaccinated with autologous DC, pulsed with KLH as an adjuvant and immune monitoring tool, and various tumor-associated antigens in an ongoing clinical trial. These patients received 3 DC vaccines intravenously and intradermally with biweekly intervals. PBMCs obtained 2 weeks after the second vaccination were used for functional studies, and DCs were cultured from autologous apheresis material collected before the start of vaccination therapy. To examine the stimulatory capacity of PD-L knockdown DCs on MiHA-specific T-cell responses, we used PBMCs obtained from 3 leukemia patients who developed a MiHA-specific CD8+ T-cell response after DLI. Patient UPN543 suffered from an acute myeloid leukemia (AML) and developed a strong LRH-1–specific CD8+ T-cell response upon preemptive DLI. Then, the patient remained in complete clinical and cytogenetic remission until 3 years after DLI, whereupon extramedullary relapses developed without leukemic involvement in the bone marrow.25,26 Patient UPN640 had a blast crisis (BC) chronic myeloid leukemia (CML) and was treated with preemptive DLI after alloSCT. This resulted in a HA-1–specific CD8+ effector T-cell response. The third patient (UPN389) suffered from an accelerated-phase (AP) chronic myeloid leukemia (CML) and was successfully treated with therapeutic DLI after alloSCT. After DLI, a LRH-1–specific CD8+ T-cell response was observed, which coincided with remission of CML-AP.6,26 However, despite the long-term presence of LRH-1–specific CD8+ memory T cells, the patient relapsed 4 years after DLI. Because no apheresis material of the corresponding donors was available, DCs were cultured from apheresis material of allogeneic HLA-B7+LRH-1− or HLA-A2+HA-1−donors. All cells of healthy donors and patients were obtained after written informed consent.
Cell culture
LRH-1–specific CD8+ cytotoxic T lymphocyte (CTL) culture RP1 was isolated from CML-AP patient UPN389 and recognized the 9-mer epitope, TPNQRQNVC, in the context of HLA-B*0702. CTL RP1 was cultured as described previously.6,25 Recipient and donor Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines (LCLs) of patient UPN389 were cultured in Iscove modified Dulbecco medium (IMDM; Invitrogen) supplemented with 10% fetal calf serum (FCS; Integro).
Generation of monocyte-derived DCs
Monocytes were isolated from PBMCs via plastic adherence in tissue-culture flasks (Greiner Bio-One). Immature DCs were generated by culturing monocytes in X-VIVO-15 medium (Lonza) supplemented with 2% human serum (HS; PAA Laboratories), 500 U/mL IL-4 (Immunotools), and 800 U/mL GM-CSF (Immunotools). After 2-3 days, half of the medium was replaced with fresh X-VIVO-15/2% HS medium containing 1000 U/mL IL-4 and 1600 U/mL GM-CSF. Maturation of DCs was induced at day 6-7 by culturing 0.5 × 106 immature (electroporated) DCs/mL in 6-well plates (Costar; Corning) in X-VIVO-15/2% HS containing 500 U/mL IL-4, 800 U/mL GM-CSF, 5 ng/mL IL-1ß, 15 ng/mL IL-6, 20 ng/mL TNF-α (all Immunotools), and 1 μg/mL PGE2 (Pharmacia & Upjohn). At day 8, mature DCs were harvested and used for T-cell stimulation, unless stated otherwise.
Stealth siRNA duplexes
Three different PD-L1 and PD-L2 Stealth siRNA duplexes and 2 recommended Stealth negative control siRNA duplexes, for either low or medium GC content, were obtained from Invitrogen. The PD-L1 siRNA and PD-L2 siRNA target sequences are listed in Table 1. All siRNAs were dissolved or diluted to a concentration of 25μM in diethyl pyrocarbonate (DEPC) water (Invitrogen) and subsequently stored at −20°C.
DC electroporation
Immature DCs were harvested at days 6-7, then washed once with Hanks balanced salt solution (HBSS; Lonza) and twice with phenol red free Optimem buffer (Gibco Invitrogen). Cells were resuspended in Optimem buffer and ≤ 7.5 × 106 cells were transferred to a 0.4-cm gene-pulser cuvette (Bio-Rad). Subsequently, 0.25 nmol of a single siRNA or, in the case of double knockdown, 0.125 nmol per siRNA, was added. DCs were electroporated at 300 V, 150 μF using a Bio-Rad Genepulser II. Directly after electroporation, cells were resuspended in prewarmed phenol red free X-VIVO-15/7% HS and incubated at 37°C. After 1 hour, phenol red free medium was replaced with conventional maturation medium. Cells were cultured for 1 to 5 days.
RNA isolation and real-time quantitative reverse-transcription PCR
Total RNA was isolated from mature PD-L knockdown DCs using the Quick-RNA–miniprep (Zymo Research Corporation). cDNA synthesis and polymerase chain reaction (PCR) amplification were performed as previously described.27,28 The hydroxymethylbilane synthase (HMBS) housekeeping gene was used to normalize gene expression. One microliter of cDNA was amplified in a 50-μL reaction mixture containing 1.25 U AmpliTaq Gold (Applied Biosystems), 300nM gene-specific forward and reverse primer, 100-300nM gene-specific Taqman probe (100nM for PD-L2 and 300nM for PD-L1 and HMBS), 250μM of each deoxynucleotide triphosphate (dNTP), 5mM MgCl2, and 1× Taqman PCR buffer (Applied Biosystems). The following gene-specific primers and Taqman probes were used: PD-L1; PD-L1 FW 5′-CATCTTATTATGCCTTGGTGTAGCA-3′, PD-L1 RV 5′-GGATTACGTCTCCTCCAAATGTG-3′ and PD-L1 MGB probe 5′-(TET)-ACATTCATCTTCCGTTTAAG-3′, PD-L2; PD-L2 FW 5′-CAACTTGGCTGCTTCACATTTT-3′, PD-L2 RV 5′-TGTGGTGACAGGTCTTTTTGTTGT-3′ and PD-L2 probe 5′-(TET)-TTCATAGCCACAGTGATAGCCCTAAGAAAACAACTCT-(TAMRA)-3′, HMBS; HMBS-FW 5′-GGCAATGCGGCTGCAA-3′, HMBS-RV 5′-GGGTACCCACGCGAATCAC-3′, and HMBS-probe 5′-(VIC)-CTCATCTTTGGGCTGTTTTCTTCCGCC-(TAMRA)-3′. PCR amplification was performed using an ABI Prism 7700 (Applied Biosystems), with the following PCR conditions: enzyme activation for 10 minutes at 95°C, followed by 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. PD-L1 and PD-L2 ΔCt values were normalized for HMBS by calculating ΔCt = Ctgene − CtHMBS per sample. Finally, PD-L1 and PD-L2 mRNA expression levels were quantified relative to DCs electroporated without siRNA as follows: 2−(ΔCt electroporated with siRNA − ΔCt electroporated without siRNA).
Flow cytometry
Phenotype and maturation state of DCs was analyzed by staining with the following antibodies: anti-CD14 (clone TÜK4, Dako), anti-CD80 (clone MAB104), anti-CD83 (clone HB15a), anti-CD86 (clone HA5.2B7, all from Beckman Coulter), anti-PD-L1 (clone MIH1), anti-PD-L2 (clone MIH18, both from Becton Dickinson), and isotype controls IgG1 FITC/PE dual-color control (Dako) and IgG2b phycoerythrin (PE; Beckman Coulter). LRH-1– and HA-1–specific T cells were detected by staining cell suspensions with PE-labeled LRH-1/HLA-B7 tetramers containing peptide TPNQRQNVC and PE-labeled HA-1/HLA-A2 tetramers containing peptide VLHDDLLEA, respectively. Tetramers were kindly provided by Prof Dr Fred Falkenburg (Department of Hematology, Leiden University Medical Center, Leiden, The Netherlands). T-cell cultures were incubated with 1.5-2 μg/mL tetramer for 15 minutes at room temperature. Subsequently, cells were labeled with the appropriate concentrations of anti-CD8 (ProImmune) and anti-CD3 (Beckman Coulter) for 30 minutes at 4°C. After washing with phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA; Sigma-Aldrich), cells were resuspended in washing buffer containing 0.1% 7-amino-actinomycin D (7-AAD; Sigma-Aldrich). Cells were analyzed using the Coulter FC500 flow cytometer (Beckman Coulter).
Allogeneic mixed lymphocyte reactions (MLRs)
Allogeneic CD4+ and CD8+ T cells were isolated from nonadherent or complete PBMC fractions by direct magnetic labeling with the appropriate MicroBeads (Miltenyi Biotec), following the manufacturer's instructions. Cells were resuspended in IMDM/10% HS to a concentration of 0.5 × 106 cells/mL and stimulated with maturated PD-L knockdown DCs at T cell:DC ratios ranging from 1:0.1 to 1:0.0125. These cocultures were performed in 48-well plates (Cellstar; Greiner Bio-one) in a total volume of 1 mL IMDM/10% HS. After 1-5 days of coculture, supernatant was harvested for cytokine analysis. At day 5, cells were resuspended and transferred to 96-well round-bottomed plates (Costar; Corning). Subsequently, 0.5μCi [3H]-thymidine (PerkinElmer) was added to each well. After overnight incubation, [3H]-thymidine incorporation was measured using a 1205 Wallac Betaplate counter (PerkinElmer).
KLH-specific T-cell activation assays
Autologous monocyte-derived DCs were cultured from apheresis material of MM patients as described above. At day 3, DCs were pulsed with or without 50 μg/mL KLH (Vacmune; Biosyn Corporation). Patient PBMCs containing KLH-specific T cells were thawed, resuspended in IMDM/10% HS to a concentration of 0.5 × 106 cells/mL, and mixed with autologous 2-day mature PD-L knockdown DCs at a T cell:DC ratio of 1:0.05. These cocultures were performed in 6-fold in 96-well round-bottomed plates (Costar; Corning) in a total volume of 200 μL. After 1 and 5 days of coculture, supernatant was harvested for cytokine analysis. At day 5, proliferation was analyzed using [3H]-thymidine, as described before.
MiHA-specific T-cell expansion assays
LRH-1–specific CTL RP1 was resuspended in IMDM/10% HS to a concentration of 0.05 × 106 cells/mL and plated in 96-well round-bottomed plates (Costar; Corning). HLA-B7+ PD-L knockdown DCs were loaded with or without 10μM LRH-1 peptide TPNQRQNVC for 30 minutes at room temperature and, subsequently, cocultured with CTL at a stimulation ratio of 1:1 in a total volume of 200 μL. After 1 day, 100 μL of supernatant was harvested, and fresh IMDM/10% HS containing 100 U/mL IL-2 (Chiron) was added. At day 4, CTL proliferation was analyzed using [3H]-thymidine, as described before, and supernatant was analyzed for cytokine levels.
MiHA-specific CD8+ memory T cells present in PBMCs from patients UPN543, UPN640, and UPN389 were stimulated for 1-3 consecutive weeks ex vivo with unloaded or MiHA peptide-loaded PD-L knockdown DCs. Mature allogeneic PD-L knockdown or control DCs, cultured from apheresis material of a HLA-B7+LRH-1− or HLA-A2+HA-1− donor, were loaded with or without 5μM MiHA peptide (LRH-1: TPNQRQNVC; HA-1: VLHDDLLEA) for 30 minutes at room temperature. PBMCs and DCs were subsequently cocultured at a ratio of 1:0.1 in 2 mL IMDM/10% HS in 24-well plates (Costar; Corning). After 5 days, CD4+ T cells were depleted from the cultures by direct magnetic labeling with CD4 IMag beads (Becton Dickinson). CD4-depleted fractions were resuspended in IMDM/10% HS supplemented with 50 U/mL IL-2 and 5 ng/mL IL-15 (Immunotools). At day 6 or 7, cells were harvested and counted, and the percentage of LRH-1– or HA-1–specific CD8+ T cells was determined by flow cytometry.
CD107a degranulation assay
PBMC cultures stimulated for 2 consecutive weeks with LRH-1 peptide-loaded PD-L knockdown DCs were used in a CD107a secretion assay to determine functional recognition of the LRH-1 antigen by measuring CTL degranulation. As target cells, we used the corresponding recipient EBV-LCLs and donor EBV-LCLs loaded with or without 5μM LRH-1 peptide. Briefly, 2 × 105 cells of each T-cell culture were stimulated with target cells at an effector:target ratio of 1:1 in a total volume of 500 μL of IMDM/10% HS supplemented with 25 U/mL of IL-2 and 6.5 μL of anti-CD107a (Becton Dickinson). After overnight coculture, the percentage of CD107a+ cells was determined within the LRH-1-tetramer+ T-cell population using flow cytometry.
Cytokine analyses
IFN-γ and granzyme B levels in culture supernatants were analyzed using enzyme-linked immunosorbent assays (ELISAs; IFN-γ: Pierce Endogen; granzyme B: MabTech). Release of IL-2, IL-4, IL-5, TNF-α, and IFN-γ by the T cells was simultaneously determined in pooled supernatant using a Th1/Th2 BD cytometric bead array (Becton Dickinson), following the manufacturer's protocol, and measured using flow cytometry.
Statistical analysis
Statistical significance of differences between PD-L knockdown DCs and medium GC siRNA control DCs were analyzed using one-way analysis of variance (ANOVA), followed by a Bonferroni posthoc test. P values < .05 were considered significant.
Results
Efficient, specific, and long-lasting siRNA–mediated knockdown of PD-L1 and PD-L2 on DCs
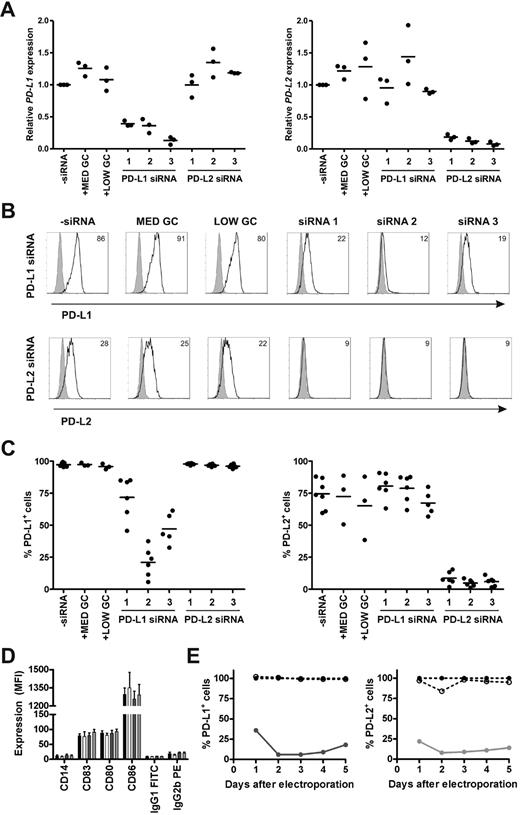
To determine the efficacy and specificity of 3 different siRNAs in silencing PD-L1 and PD-L2 expression on monocyte-derived DCs, we studied the magnitude, off-target effects, and duration of knockdown on mRNA and protein level. Immature DCs were electroporated with or without 0.25 nmol siRNA and subsequently cultured in maturation medium containing IL-1β, IL-6, TNF-α, and PGE2. All 3 PD-L1 siRNAs appreciably reduced PD-L1 mRNA levels at 2 days after electroporation, while the negative control siRNAs had no effect (Figure 1A). Similarly, all 3 PD-L2 siRNAs efficiently knocked down PD-L2 mRNA expression to the same extent. To validate knockdown on the protein level, we analyzed PD-L1 and PD-L2 surface expression on siRNA-electroporated DCs 2 days after maturation. PD-L1 siRNA number 2 induced the most pronounced reduction of PD-L1 cell-surface expression, resulting in an average of 21% PD-L1+ DCs (n = 6; Figure 1B-C). In the case of PD-L2, all 3 siRNAs were equally effective, resulting in < 10% PD-L2+ DCs. Therefore, we decided to continue with PD-L1 and PD-L2 siRNA number 2 and the accompanying medium GC-negative control siRNA. To verify that our siRNAs of interest did not affect DC maturation or have other off-target effects, protein expression levels of various maturation and costimulatory molecules were examined 2 days after electroporation. None of the molecules were affected by the siRNAs tested (Figure 1D). Finally, we determined the duration of the siRNA-mediated knockdown effects by assessing PD-L1 and PD-L2 protein expression over time upon DC electroporation. After 2 days, PD-L1 and PD-L2 were both maximally down-regulated by the respective siRNAs and expression remained low for at least 3 more days (Figure 1E). Collectively, these data show that siRNA electroporation of DCs results in efficient, specific, and long-lasting silencing of PD-L1 and PD-L2.
Efficacy, specificity, and duration of siRNA-mediated PD-L1 and PD-L2 silencing on DCs. Immature DCs were electroporated with 0.25 nmol PD-L1, PD-L2, or negative control (MED GC vs LOW GC) siRNA duplexes and subsequently cultured in maturation medium containing IL-1β, IL-6, TNF-α, and PGE2 for 2 days (A-D) or 1-5 days (E). (A) PD-L1 and PD-L2 mRNA expression was measured and subsequently normalized for HMBS expression using RT Q-PCR. PD-L mRNA expression in DCs electroporated without siRNA was set to 1. (B) PD-L1 and PD-L2 protein expression (black lines), compared with isotype control (gray histograms), was analyzed using flow cytometry. The numbers in the plots represent the mean fluorescence intensity (MFI). The data of 1 representative donor of 6 is shown. (C) Percentage of PD-L1+ or PD-L2+ DCs was determined for 3-6 donors by flow cytometry. (D) Expression of maturation and costimulatory molecules by electroporated DCs was analyzed using flow cytometry. The bars represent DCs electroporated without siRNA (black), with MED GC control siRNA (white), PD-L1 siRNA 2 (dark gray), or PD-L2 siRNA 2 (light gray). Data are expressed as mean ± SEM of 3-6 donors. (E) Percentage of PD-L1+ or PD-L2+ DCs over time was determined by flow cytometry. The percentage of PD-L+ DCs electroporated without siRNA was set to 100%. The lines represent DCs electroporated without siRNA (dotted line, ●), with MED GC control siRNA (dotted line, ○), PD-L1 siRNA 2 (solid dark gray line), or PD-L2 siRNA 2 (solid light gray line). The data are representative of 2 independent experiments on 2 different donors.
Efficacy, specificity, and duration of siRNA-mediated PD-L1 and PD-L2 silencing on DCs. Immature DCs were electroporated with 0.25 nmol PD-L1, PD-L2, or negative control (MED GC vs LOW GC) siRNA duplexes and subsequently cultured in maturation medium containing IL-1β, IL-6, TNF-α, and PGE2 for 2 days (A-D) or 1-5 days (E). (A) PD-L1 and PD-L2 mRNA expression was measured and subsequently normalized for HMBS expression using RT Q-PCR. PD-L mRNA expression in DCs electroporated without siRNA was set to 1. (B) PD-L1 and PD-L2 protein expression (black lines), compared with isotype control (gray histograms), was analyzed using flow cytometry. The numbers in the plots represent the mean fluorescence intensity (MFI). The data of 1 representative donor of 6 is shown. (C) Percentage of PD-L1+ or PD-L2+ DCs was determined for 3-6 donors by flow cytometry. (D) Expression of maturation and costimulatory molecules by electroporated DCs was analyzed using flow cytometry. The bars represent DCs electroporated without siRNA (black), with MED GC control siRNA (white), PD-L1 siRNA 2 (dark gray), or PD-L2 siRNA 2 (light gray). Data are expressed as mean ± SEM of 3-6 donors. (E) Percentage of PD-L1+ or PD-L2+ DCs over time was determined by flow cytometry. The percentage of PD-L+ DCs electroporated without siRNA was set to 100%. The lines represent DCs electroporated without siRNA (dotted line, ●), with MED GC control siRNA (dotted line, ○), PD-L1 siRNA 2 (solid dark gray line), or PD-L2 siRNA 2 (solid light gray line). The data are representative of 2 independent experiments on 2 different donors.
PD-L knockdown DCs show increased capacity to stimulate cytokine production in allogeneic MLR assays
To investigate whether knockdown of PD-L1 and/or PD-L2 using the selected siRNAs (ie, PD-L1 and PD-L2 target sequence no. 2 in Table 1) improved the stimulatory capacity of DCs, allogeneic T cells were cocultured for 5 days with mature PD-L knockdown DCs. Thereafter, T-cell proliferation and cytokine production was analyzed. A strong, dose-dependent increase in IFN-γ production was observed upon stimulation with PD-L knockdown DC, but PD-L knockdown DCs were not capable of improving T-cell proliferation of allogeneic CD4+ or CD8+ T cells (Figure 2A-B). For CD4+ T cells, IFN-γ levels were enhanced up to 7-fold by PD-L1 knockdown DCs, whereas PD-L2 knockdown DCs only modestly improved IFN-γ production (Figure 2B). Interestingly, stimulation with PD-L1/L2 double-knockdown DCs resulted in a 10-fold higher IFN-γ production, indicating a synergistic effect when PD-L1 and PD-L2 are knocked down simultaneously. Although CD8+ T cells produced less IFN-γ than CD4+ T cells, a still considerable elevation of IFN-γ levels was observed upon stimulation with PD-L knockdown DC. Especially, PD-L1 single- and PD-L1/L2 double-knockdown DCs improved IFN-γ levels up to 3-fold, while the increase by PD-L2 single knockdown DCs was again modest.
PD-L knockdown DCs show enhanced stimulation of allogeneic T cells. Immature DCs were electroporated with 0.25 nmol of each single siRNA or 0.125 nmol of each siRNA in case of double knockdown. After 2 days of maturation, DCs were mixed with allogeneic T cells. (A) At day 5 of coculture, [3H]-thymidine was added to the culture, and the following day, T-cell proliferation capacity was determined by measuring [3H]-thymidine incorporation. Data are depicted as mean ± SD and are representative for 2 different donors. CPM = counts per minute. (B) IFN-γ levels were measured in the supernatant at day 5 of coculture using ELISA. Data are depicted as mean ± SD and are representative for 3 different donors. (C) Cytokines produced by CD4+ and CD8+ T cells of the same donor stimulated with allogeneic DCs at a ratio of 1:0.1 were analyzed simultaneously at days 1-5 of the coculture. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, **P < .01, ***P < .001.
PD-L knockdown DCs show enhanced stimulation of allogeneic T cells. Immature DCs were electroporated with 0.25 nmol of each single siRNA or 0.125 nmol of each siRNA in case of double knockdown. After 2 days of maturation, DCs were mixed with allogeneic T cells. (A) At day 5 of coculture, [3H]-thymidine was added to the culture, and the following day, T-cell proliferation capacity was determined by measuring [3H]-thymidine incorporation. Data are depicted as mean ± SD and are representative for 2 different donors. CPM = counts per minute. (B) IFN-γ levels were measured in the supernatant at day 5 of coculture using ELISA. Data are depicted as mean ± SD and are representative for 3 different donors. (C) Cytokines produced by CD4+ and CD8+ T cells of the same donor stimulated with allogeneic DCs at a ratio of 1:0.1 were analyzed simultaneously at days 1-5 of the coculture. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, **P < .01, ***P < .001.
To get more insight into the levels and kinetics of various cytokines produced by the different T-cell subsets upon stimulation with PD-L knockdown DCs, coculture supernatant was harvested over time and analyzed. Early after stimulation with DCs, allogeneic T cells started producing IL-2, while production of IFN-γ, TNF-α, and IL-5 initiated later (Figure 2C). No IL-4 was produced (data not shown). Overall, highest cytokine levels were observed in cocultures with CD4+ T cells. Furthermore, over time, PD-L knockdown DCs evidently elevated IL-2, IFN-γ, TNF-α, and IL-5 production by CD4+ as well as CD8+ T cells. For all cytokines, the highest levels were obtained after stimulation with the PD-L1/L2 double-knockdown DCs.
Altogether, these results demonstrate that stimulation with PD-L knockdown DCs has no effect on T-cell proliferation, but profoundly enhances the cytokine production capacity of stimulated T cells in a primary allogeneic MLR.
KLH-specific T-cell responses are strongly augmented by PD-L knockdown DCs
To examine the stimulatory capacity of PD-L-silenced DCs in secondary responses by antigen-experienced T cells, we cultured PBMCs containing primed KLH-specific T cells together with autologous KLH-pulsed PD-L knockdown DCs. After 1-5 days, KLH-specific proliferation and cytokine production of DC-stimulated T cells was studied. Interestingly, the proliferative capacity of primed KLH-specific T cells could be significantly boosted with PD-L knockdown DCs (Figure 3A). Using PBMCs from MM patient 1, all 3 knockdown DC types increased KLH-specific T-cell proliferation, whereas in patient 2, significant induction of proliferation was only seen upon stimulation with PD-L1/PD-L2 double-knockdown DCs. In accordance with the results found in the allogeneic MLR setting, stimulation with PD-L knockdown DCs resulted in increased cytokine production by KLH-specific T cells (Figure 3B). Stimulation with PD-L1 knockdown DCs resulted in 2-5× higher IFN-γ levels than after coculture with control siRNA DCs. Despite the modest elevation of IFN-γ production after stimulation with PD-L2-silenced DCs, an evident synergistic augmentation (ie, 8-14-fold) of IFN-γ levels was observed when KLH-specific T cells were stimulated with PD-L1/L2 double-knockdown DCs. Already 1 day after stimulation with KLH-pulsed DCs, IL-2 production was markedly elevated by all PD-L knockdown DC types (Figure 3C). Again, the most evident enhancement was seen for PD-L1 single and PD-L1/L2 dual knockdown DCs. Moreover, in PD-L1 single and PD-L1/L2 double-knockdown DC-stimulated cultures, IL-2 levels remained considerably higher up to 5 days after stimulation of the KLH-specific T cells. These data demonstrate that KLH-pulsed PD-L knockdown DCs augment proliferation and strongly increase cytokine production by primed KLH-specific T cells of DC-vaccinated cancer patients.
PD-L siRNA-silenced DCs show an increased capacity to stimulate KLH-specific T-cell proliferation and cytokine production in vaccinated cancer patients. Autologous PD-L knockdown DCs pulsed with or without KLH were cocultured with PBMCs from vaccinated MM patients at a stimulation ratio of 0.1:1. (A) At day 5, [3H]-thymidine was added to the culture, and the following day, T-cell proliferation capacity was determined by measuring [3H]-thymidine incorporation. Representative data of 2 of 5 patients are shown, and bars are depicted as mean ± SD. CPM indicates counts per minute. (B) IFN-γ levels were measured in the supernatant at day 5 of the coculture. Representative data of 2 of 5 patients are shown, and bars are depicted as mean ± SD. (C) IL-2 levels were measured in coculture supernatant of days 1 and 5. Representative data of 2 of 5 patients are shown. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, **P < .01, ***P < .001.
PD-L siRNA-silenced DCs show an increased capacity to stimulate KLH-specific T-cell proliferation and cytokine production in vaccinated cancer patients. Autologous PD-L knockdown DCs pulsed with or without KLH were cocultured with PBMCs from vaccinated MM patients at a stimulation ratio of 0.1:1. (A) At day 5, [3H]-thymidine was added to the culture, and the following day, T-cell proliferation capacity was determined by measuring [3H]-thymidine incorporation. Representative data of 2 of 5 patients are shown, and bars are depicted as mean ± SD. CPM indicates counts per minute. (B) IFN-γ levels were measured in the supernatant at day 5 of the coculture. Representative data of 2 of 5 patients are shown, and bars are depicted as mean ± SD. (C) IL-2 levels were measured in coculture supernatant of days 1 and 5. Representative data of 2 of 5 patients are shown. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, **P < .01, ***P < .001.
MiHA-specific CTL expansion and function is enhanced by PD-L silenced DCs
Recently, we have observed that activated LRH-1–specific CD8+ T cells express PD-1, and that in vitro antibody blockade of PD-1 signaling results in improved stimulation of LRH-1–specific CD8+ T cells by PD-L1–expressing DCs and myeloid leukemia cells (Norde et al, manuscript in preparation). To investigate the effect of PD-L silencing on MiHA-specific CD8+ T-cell expansion and functionality initiated by DCs, we stimulated LRH-1–specific CTL clone RP1 with PD-L knockdown DCs loaded with or without LRH-1 peptide for 1-4 days. After DC stimulation, we measured the proliferative capacity as well as production of IFN-γ and granzyme B by LRH-1–specific CTL RP1. After 4 days of coculture, LRH-1–dependent proliferation of CTL RP1 was observed (Figure 4A). Single PD-L1 and dual PD-L1/PD-L2 knockdown significantly enhanced DC-mediated CTL proliferation, while the effect of PD-L2 knockdown was only modest. Furthermore, we observed increased IFN-γ production by LRH-1–specific CTL after stimulation with all 3 LRH-1 peptide-loaded PD-L knockdown DCs (Figure 4B). Moreover, production of granzyme B by CTL RP1 was augmented by PD-L1–silenced DCs upon peptide recognition (Figure 4C). These results indicate that PD-L–silenced DCs are able to increase proliferation rate and degranulation capacity of MiHA-specific CTLs.
Expansion and function of MiHA-specific CTL RP1 is augmented by PD-L knockdown DCs. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+ DCs were loaded with or without 10μM LRH-1 peptide and cocultured with MiHA-specific CTL RP1 at a stimulation ratio of 1:1. PD-L knockdown resulted in < 20% PD-L1+ and/or < 1% PD-L2+ DC. (A) After 4 days, proliferation was determined following overnight [3H]-thymidine incorporation. Data of 1 of 2 independent experiments are shown, and bars are depicted as mean ± SD. CPM = counts per minute. (B) IFN-γ and (C) granzyme B levels were measured in CTL RP1 supernatant obtained at day 1 of coculture. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, ***P < .001.
Expansion and function of MiHA-specific CTL RP1 is augmented by PD-L knockdown DCs. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+ DCs were loaded with or without 10μM LRH-1 peptide and cocultured with MiHA-specific CTL RP1 at a stimulation ratio of 1:1. PD-L knockdown resulted in < 20% PD-L1+ and/or < 1% PD-L2+ DC. (A) After 4 days, proliferation was determined following overnight [3H]-thymidine incorporation. Data of 1 of 2 independent experiments are shown, and bars are depicted as mean ± SD. CPM = counts per minute. (B) IFN-γ and (C) granzyme B levels were measured in CTL RP1 supernatant obtained at day 1 of coculture. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, ***P < .001.
PD-L knockdown DCs effectively boost ex vivo expansion and function of MiHA-specific CD8+ effector and memory T cells
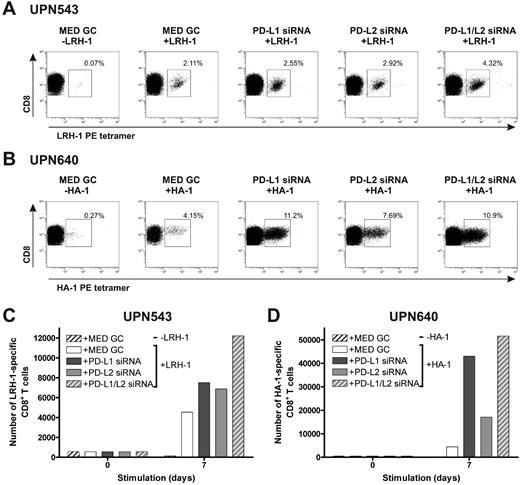
To investigate the effect of stimulation with MiHA-presenting, PD-L–silenced DCs on MiHA-specific CD8+ effector and memory T-cell proliferation, we used PBMCs from 3 different leukemia patients collected early after SCT and DLI or during relapsed disease. First, we cultured PBMCs of patients UPN543 and UPN640, both obtained 7 months after DLI during the effector response, with MiHA-presenting, PD-L–silenced DCs for 1 week. At start, PBMCs contained 0.12% LRH-1–specific and 0.14% HA-1–specific CD8+ T cells, respectively. In both patients, we observed augmented percentages of MiHA-specific CD8+ T cells after stimulation with MiHA-loaded, PD-L–silenced DCs, compared with control DCs (Figure 5A-B). Although the best effects were observed upon stimulation with PD-L1/L2 double-knockdown DCs, all PD-L knockdown DC types evidently increased the number of LRH-1–specific CD8+ T cells of patient UPN543 after 1 week of culture ranging from 1.5- to 2.7-fold (Figure 5C). In the case of patient UPN640, the effects were even more pronounced. One-week culture with PD-L knockdown DCs resulted in considerably augmented expansion of HA-1–specific CD8+ T cells of up to 12-fold for PD-L1/L2 double-knockdown DCs (Figure 5D).
MiHA-specific CD8+ effector T-cell expansion can be enhanced by PD-L–silenced DCs. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+LRH-1− or HLA-A2+HA-1− DCs were loaded with or without 5μM MiHA peptide. PD-L knockdown resulted in < 17% PD-L1+ and/or < 5% PD-L2+ DCs. Patient PBMCs, containing MiHA-specific CD8+ effector T cells, stimulated with PD-L knockdown DCs at a ratio of 1:0.1, were screened after 1 week for tetramer+ CD8+ T cells using flow cytometry. The numbers in the FACS plots represent the percentage of (A) LRH-1– (UPN543) or (B) HA-1–specific (UPN640) CD8+ T cells in the total CD3+CD8+ T-cell population. Total numbers of LRH-1– (C) or HA-1–specific (D) CD8+ T cells obtained after 1 week of stimulation with PD-L knockdown DCs loaded with or without MiHA peptide.
MiHA-specific CD8+ effector T-cell expansion can be enhanced by PD-L–silenced DCs. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+LRH-1− or HLA-A2+HA-1− DCs were loaded with or without 5μM MiHA peptide. PD-L knockdown resulted in < 17% PD-L1+ and/or < 5% PD-L2+ DCs. Patient PBMCs, containing MiHA-specific CD8+ effector T cells, stimulated with PD-L knockdown DCs at a ratio of 1:0.1, were screened after 1 week for tetramer+ CD8+ T cells using flow cytometry. The numbers in the FACS plots represent the percentage of (A) LRH-1– (UPN543) or (B) HA-1–specific (UPN640) CD8+ T cells in the total CD3+CD8+ T-cell population. Total numbers of LRH-1– (C) or HA-1–specific (D) CD8+ T cells obtained after 1 week of stimulation with PD-L knockdown DCs loaded with or without MiHA peptide.
Next, we investigated the effect of repetitive stimulations with MiHA-presenting PD-L–silenced DCs on MiHA-specific CD8+ memory T cells. For this, we used PBMCs from CML-AP patient UPN389 and AML patient UPN543 containing, respectively, 0.06% and 0.09% LRH-1–specific CD8+ T cells, collected at 64 (UPN389) and 71 (UPN543) months post-DLI during relapsed disease. These PBMCs were stimulated for 2 or 3 consecutive weeks with LRH-1 peptide-loaded DCs, and the number of LRH-1–specific CD8+ T cells was weekly determined by flow cytometry. After stimulation with LRH-1 peptide-loaded control DCs, a specific increase in the percentage of LRH-1-tetramer+ CD8+ T cells of 8% was observed over time in patient UPN389 (Figure 6A). Interestingly, PD-L1 single- and PD-L1/PD-L2 double-knockdown DCs improved LRH-1–specific CD8+ memory T-cell expansion up to 12% and 29%, respectively. Surprisingly, lower percentages of LRH-1-specific CD8+ T cells were detected upon stimulation with LRH-1 peptide-loaded PD-L2 knockdown DCs. Because absolute T-cell numbers and the percentage of CD8+ T cells were higher in PD-L–silenced DC-stimulated cultures compared with T-cell cultures stimulated with control DCs, we also calculated the cumulative number of LRH-1–specific CD8+ T cells over time (Figure 6B). In the first week, the fold expansion upon stimulation with PD-L1 single and PD-L1/L2 double-knockdown DCs was, respectively, 1.6 and 4.7× higher than that of LRH-1 peptide-loaded control DCs. Ultimately, stimulation with PD-L1 single or PD-L1/L2 double-knockdown DCs resulted in a 2.8 and 23.3× higher number of LRH-1–specific CD8+ T cells by the end of week 3.
PD-L knockdown DCs boost the proliferative capacity and function of MiHA-specific CD8+ memory T cells. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+LRH-1− DCs were loaded with or without 5μM LRH-1 peptide. PD-L knockdown resulted in < 5% PD-L1+ and/or < 2% PD-L2+ DCs. (A) PBMCs of patient UPN389, consecutively stimulated with PD-L knockdown DCs at a ratio of 1:0.1, were weekly screened for tetramer+ CD8+ T cells using flow cytometry. The numbers in the FACS plots represent the percentage of LRH-1–specific CD8+ T cells in the total CD3+CD8+ T-cell population. Data are representative for 2 independent experiments. (B) Cumulative numbers of LRH-1–specific CD8+ T cells of patient UPN389 obtained after 3 consecutive stimulations with PD-L knockdown DCs loaded with or without LRH-1 peptide. (C) Percentage of LRH-1–specific CD8+ T cells in PBMCs of patient UPN543. PBMCs were consecutively stimulated with PD-L knockdown DCs at a ratio of 1:0.1 and weekly screened for tetramer+ CD8+ T cells using flow cytometry. (D) Cumulative numbers of LRH-1–specific CD8+ T cells of patient UPN543 obtained after 2 consecutive stimulations with PD-L knockdown DCs loaded with or without LRH-1 peptide. (E) Degranulation of LRH-1–specific CD8+ T cells of patient UPN389, stimulated for 2 weeks with LRH-1 peptide-loaded PD-L knockdown DC, was measured by staining for CD107a during overnight stimulation with different target cells. PBMCs were cultured 1:1 with LRH-1− donor EBV-LCL, LRH-1 peptide-loaded donor EBV-LCL, LRH-1+ recipient EBV-LCL, or in medium. ND indicates not determined.
PD-L knockdown DCs boost the proliferative capacity and function of MiHA-specific CD8+ memory T cells. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+LRH-1− DCs were loaded with or without 5μM LRH-1 peptide. PD-L knockdown resulted in < 5% PD-L1+ and/or < 2% PD-L2+ DCs. (A) PBMCs of patient UPN389, consecutively stimulated with PD-L knockdown DCs at a ratio of 1:0.1, were weekly screened for tetramer+ CD8+ T cells using flow cytometry. The numbers in the FACS plots represent the percentage of LRH-1–specific CD8+ T cells in the total CD3+CD8+ T-cell population. Data are representative for 2 independent experiments. (B) Cumulative numbers of LRH-1–specific CD8+ T cells of patient UPN389 obtained after 3 consecutive stimulations with PD-L knockdown DCs loaded with or without LRH-1 peptide. (C) Percentage of LRH-1–specific CD8+ T cells in PBMCs of patient UPN543. PBMCs were consecutively stimulated with PD-L knockdown DCs at a ratio of 1:0.1 and weekly screened for tetramer+ CD8+ T cells using flow cytometry. (D) Cumulative numbers of LRH-1–specific CD8+ T cells of patient UPN543 obtained after 2 consecutive stimulations with PD-L knockdown DCs loaded with or without LRH-1 peptide. (E) Degranulation of LRH-1–specific CD8+ T cells of patient UPN389, stimulated for 2 weeks with LRH-1 peptide-loaded PD-L knockdown DC, was measured by staining for CD107a during overnight stimulation with different target cells. PBMCs were cultured 1:1 with LRH-1− donor EBV-LCL, LRH-1 peptide-loaded donor EBV-LCL, LRH-1+ recipient EBV-LCL, or in medium. ND indicates not determined.
In addition, for AML patient UPN543, we observed a specific increase in the percentage of LRH-1-tetramer+ CD8+ T cells to 0.7% upon repetitive stimulations with LRH-1 peptide-loaded DCs (Figure 6C). Silencing of PD-1 ligands on these DCs resulted in improved percentages of LRH-1–specific CD8+ T cells of 2.5% and 10% for PD-L single and PD-L1/L2 double-knockdown DCs, compared with control DCs (0.7%). In absolute numbers, the advantage of PD-L knockdown DCs over control DCs in augmenting LRH-1–specific CD8+ T-cell expansion is even more evident (Figure 6D). Eventually, this resulted in 13.3, 26.7, and 61× higher numbers of tetramer+ CD8+ T cells by PD-L1, PD-L2, and PD-L1/L2 double-knockdown DCs, respectively.
Finally, we studied the functional capacity of the expanded LRH-1–specific CD8+ T cells of patient UPN389 after 2 weeks of stimulation with LRH-1 peptide-loaded PD-L knockdown or control DCs. These DC-stimulated CD8+ T-cell cultures were incubated overnight with different target cells in the presence of anti-CD107a to determine the percentage of degranulating CD107a+ LRH-1–specific CD8+ T cells. We observed that stimulation with recipient EBV-LCL and LRH-1 peptide-loaded donor EBV-LCL resulted in > 75% CD107a+ LRH-1–specific CD8+ T cells (Figure 6E), whereas stimulation with donor EBV-LCL or medium resulted in < 25% nonspecific degranulation of the LRH-1–specific CD8+ T cells. A slight difference in degranulation capacity was seen between the LRH-1–specific CD8+ T cells stimulated with PD-L knockdown DCs, in comparison to control DCs. Together, these results demonstrate that MiHA-specific CD8+ effector and memory T-cell expansion can be efficiently boosted using PD-L knockdown DCs, and that these expanded MiHA-specific CD8+ T cells have the capacity to degranulate upon recognition of MiHA+ target cells.
Discussion
Alloreactive CD8+ T cells targeting MiHA on malignant cells of the recipient play a key role in GVT immunity after alloSCT and DLI. However, MiHA-specific CD8+ T-cell responses induced posttransplantation are in a substantial number of patients not sufficient to sustain complete remission of the malignant disease. It is evident that distinct mechanisms are involved in dampening antitumor T-cell responses, which may allow malignant cells to escape immune destruction. Among these mechanisms, T-cell inhibition or even exhaustion due to signaling of the PD-1/PD-L pathway may contribute to abrogation of immune responses by limiting the expansion and functionality of CD8+ T cells.12,,,,,–18 Recently, we showed that MiHA+ leukemia can relapse without inducing MiHA-specific CD8+ memory T-cell expansion, suggesting that these memory T cells are either inactive or are not activated by MiHA-presenting APC.6,25 Furthermore, we found that like virus-specific CD8+ T cells in chronic viral infections and tumor-infiltrating T cells in solid tumors, the PD-1/PD-L pathway is involved in the impairment of MiHA-specific CD8+ T-cell function (Norde et al, manuscript in preparation).
DC-based vaccination is an attractive strategy to prevent or treat recurrence of tumor cells by boosting MiHA-specific CD8+ T-cell immunity.26 Although results of DC vaccination therapy in cancer patients are promising, the overall clinical outcome is not satisfactory yet.20 Previously, we have shown that mature DCs have the potential to revive impaired MiHA-specific memory T cells from relapsed leukemia patients.26 However, mature DCs do not only express costimulatory molecules, but also highly express PD-L1 and PD-L2, which may inhibit their stimulatory capacity upon encounter of PD-1+ alloreactive CD8+ T cells. Here, we investigated whether PD-L1 and PD-L2 knockdown in DCs results in improved T-cell stimulation. We silenced PD-L1 and PD-L2 expression on monocyte-derived DCs using Stealth Select siRNA duplexes, which have high specificity, longevity and stability, and limited induction of nonspecific cellular stress-response pathways. Optimization experiments revealed that electroporation of iDCs, compared with mDCs, resulted in more efficient siRNA-mediated knockdown of PD-L2 and, especially, PD-L1 (data not shown). Therefore, we decided to continue with iDC electroporation followed by 2 days of maturation using the conventional cytokine cocktail, containing IL-1β, IL-6, TNF-α, and PGE2. Testing of 3 different siRNA sequences demonstrated that PD-L2 expression levels could be efficiently down-regulated by all 3 duplexes after one electroporation, while duplex number 2, located on the boundary of exons 2 and 3, was the most efficient PD-L1 siRNA sequence. Our results contrast to findings reported by Breton et al, who described that a 2-step electroporation method was necessary to obtain sufficient PD-L1 knockdown on monocyte-derived DCs.29 The difference in efficacy could be related to the used PD-L1 siRNA sequence or different electroporation conditions. Our selected PD-L1 and PD-L2 siRNAs showed specific effects for the targeted proteins and did not affect DC maturation or expression of costimulatory molecules. Furthermore, the siRNA-mediated PD-L knockdown lasted for at least 5 days. After identifying the most efficient siRNAs and electroporation protocol, we determined the stimulatory capacity of PD-L knockdown DCs in primary and secondary antigen-specific T-cell responses.
PD-L-silenced DCs significantly increased cytokine production of CD4+ and CD8+ T cells in allogeneic MLR assays; however, no improved effect of PD-L knockdown DCs on the induction of proliferative CD4+ and CD8+ T-cell responses was observed. In the early phase of T-cell stimulation, we observed increased production of IL-2 and, in the later phase, enhanced production of IFN-γ, TNF-α, and IL-5, but not IL-4. These data indicate that PD-L–silenced DCs improve CD4+ Th1 and CD8+ T-cell responses. Other studies reported increased T-cell proliferation and cytokine production during primary allogeneic MLR responses after PD-L blockade using antibodies, especially upon initiation by weaker DCs, such as iDCs and IL-10–pretreated mDCs.30 However, more profound stimulatory effects by PD-L1 and PD-L2 blockade were observed on memory and recently activated T cells during secondary immune responses.31 Therefore, we tested our PD-L knockdown DCs also in an antigen-experienced setting using PBMCs of MM patients containing KLH-specific T cells after vaccination with autologous KLH-pulsed DCs. Although effects vary to some extent, we observed, in 5 different patients, significant improvement of KLH-specific T-cell proliferation after stimulation with KLH-pulsed, PD-L–silenced DCs. Furthermore, PD-L knockdown significantly enhanced IFN-γ and IL-2 production by the KLH-specific T cells. Interestingly, the effects of PD-L2–silenced DCs were very modest, while simultaneous knockdown of PD-L1 and PD-L2 on DCs resulted in a synergistic improvement of T-cell cytokine production. A similar phenomenon was observed by Keir et al after stimulation of ovalbumin-specific CD4+ T cells with PD-L1/PD-L2−/− DCs in the presence of ovalbumin-peptide.32 This suggests that PD-L1 and PD-L2 can exert similar effects via overlapping pathways.
To investigate whether our PD-L knockdown DCs were capable of improving MiHA-specific CD8+ T-cell responses, we used LRH-1–specific CTL RP1 and PBMCs containing MiHA-specific CD8+ effector or memory T cells, collected from 3 leukemia patients early after DLI or years later during relapsed disease. In this MiHA-specific setting, LRH-1 peptide-loaded PD-L knockdown DCs improved the expansion rate of LRH-1–specific CTL RP1. In addition, IFN-γ and granzyme B levels produced by this CTL were significantly higher after stimulation with PD-L knockdown DCs. Most importantly, we demonstrated that LRH-1– and HA-1–specific CD8+ T cells could be more efficiently expanded by MiHA peptide-loaded PD-L knockdown DCs during both effector and memory T-cell responses. After the first stimulation with DCs, differences were still modest for some patient samples, but upon restimulation, PD-L knockdown DCs profoundly improved the proliferative capacity of the MiHA-specific CD8+ T cells. Surprisingly, in patient UPN389, LRH-1–specific CD8+ memory T-cell expansion was lower after stimulation with PD-L2 knockdown DCs than with control DCs. We are very intrigued by this observation, especially since PD-L1/PD-L2 double-knockdown DCs are, evidently, the best stimulator cells. It could be that under certain conditions PD-L2 acts as a costimulatory molecule by a PD-1–independent mechanism, as shown by others, and that by siRNA-mediated silencing, we inhibited LRH-1–specific T-cell activation.33,34 Another explanation could be that PD-L2 knockdown interferes with the postulated PD-L2 reverse signaling, resulting in DCs with lower stimulatory capacity.35,36 Finally, we showed that the expanded LRH-1–specific CD8+ T cells have the capacity to degranulate upon recognition of MiHA-expressing target cells, demonstrating that these cells have competent cytotoxic functions.
In conclusion, we demonstrated that efficient, specific, and long-lasting silencing of PD-L1 and PD-L2 can be achieved by single siRNA electroporation of monocyte-derived DCs. We showed that PD-L knockdown DCs profoundly boost the proliferative capacity of antigen-experienced T-cells specific for KLH and MiHA. Moreover, both in primary allogeneic MLR and secondary antigen-specific T-cell responses, PD-L knockdown DCs strongly enhance cytokine production. Together, these findings indicate that PD-L siRNA–electroporated DCs are attractive cells for improving the efficacy of MiHA-based DC vaccines to boost GVT immunity in SCT patients as well as antigen-loaded DCs in cancer patients and chronic viral infections.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Wieger Norde for helpful advice, Hanny Fredrix for technical support, and Prof Dr Fred Falkenburg and Michel Kester (Department of Hematology, Leiden University Medical Center, Leiden, The Netherlands) for providing LRH-1/HLA-B7 and HA-1/HLA-A2 tetramers.
This work was supported by a grant from the Dutch Cancer Society (KWF 2008-4018).
Authorship
Contribution: W.H. performed experiments, analyzed data, and wrote the manuscript; F.M. and N.A. assisted with the experiments; N.S. revised the manuscript and provided patient data and samples; T.W. and R.V. provided advice and revised the manuscript; and H.D. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harry Dolstra, Department of Laboratory Medicine–Laboratory of Hematology, Radboud University Nijmegen Medical Center, Geert Grooteplein 8, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: h.dolstra@labgk.umcn.nl.

![Figure 2. PD-L knockdown DCs show enhanced stimulation of allogeneic T cells. Immature DCs were electroporated with 0.25 nmol of each single siRNA or 0.125 nmol of each siRNA in case of double knockdown. After 2 days of maturation, DCs were mixed with allogeneic T cells. (A) At day 5 of coculture, [3H]-thymidine was added to the culture, and the following day, T-cell proliferation capacity was determined by measuring [3H]-thymidine incorporation. Data are depicted as mean ± SD and are representative for 2 different donors. CPM = counts per minute. (B) IFN-γ levels were measured in the supernatant at day 5 of coculture using ELISA. Data are depicted as mean ± SD and are representative for 3 different donors. (C) Cytokines produced by CD4+ and CD8+ T cells of the same donor stimulated with allogeneic DCs at a ratio of 1:0.1 were analyzed simultaneously at days 1-5 of the coculture. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-04-278739/6/m_zh89991060020002.jpeg?Expires=1769407954&Signature=MtgrrsRb9~Q0Yn8Zv90eaAcMTovq6SCZPQs4N6oQoYpwKLlfhsY2Drsc3oN8oAqj26-xNwgoT9GrATTp0AIGchyuz67OUeKnccGB4vls1iVvLDE3M3eaMF4f9tI-OL8Nv31pcC7J3caYtOL6n3Ra7kIrpYNxwMpIi99Net~67xU1JqEKlKR0Q4p9zczCkO~DqT~sppL6AlMovXqTmXGjeY-Zn8jEfxioAx8BNehwsLucks8W-EJml1fkO0paIOIPrnYi3bTYNUOhEgpZgViO2NmulkONNY0Zv7lPjJq83aUJIQk63~WkP9uqwqzghCuemQ8BanVOJKYmoxWS4PAntg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. PD-L siRNA-silenced DCs show an increased capacity to stimulate KLH-specific T-cell proliferation and cytokine production in vaccinated cancer patients. Autologous PD-L knockdown DCs pulsed with or without KLH were cocultured with PBMCs from vaccinated MM patients at a stimulation ratio of 0.1:1. (A) At day 5, [3H]-thymidine was added to the culture, and the following day, T-cell proliferation capacity was determined by measuring [3H]-thymidine incorporation. Representative data of 2 of 5 patients are shown, and bars are depicted as mean ± SD. CPM indicates counts per minute. (B) IFN-γ levels were measured in the supernatant at day 5 of the coculture. Representative data of 2 of 5 patients are shown, and bars are depicted as mean ± SD. (C) IL-2 levels were measured in coculture supernatant of days 1 and 5. Representative data of 2 of 5 patients are shown. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-04-278739/6/m_zh89991060020003.jpeg?Expires=1769407954&Signature=Oh0zSx5zc2D6rRkd-3eMGQ7INu8q2Pwp4ef089jlDEyd6uxhGDsLxwi1y-qKKQH16qlO286r6WyqirXsLVFzPltHV2vKtKef6QUJdF9fNNeDbkEcEG3vmORfEQYf6Emy~wY-0tPb~VmnN9ElUEEY3f-WpzSfj2vLOv3RXP2pmKLRltKHFDy8FN4yNJ8G-BjgyatL8EDKM7sTHJkg0RD4Ze55FOHRGowShBAv-MgyQQsWCVCPJEQHKw1CGRqeemZTDo8Ci9BtiH3SSMRrCWzbSVCPi4XQiOXfcKSPfvrt1cBcD2Q-rIS1jxx-cJusoyO7Iy7fZHk~~9liTM3pMTk08A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Expansion and function of MiHA-specific CTL RP1 is augmented by PD-L knockdown DCs. Two days after electroporation with PD-L1 and/or PD-L2 siRNA, mature HLA-B7+ DCs were loaded with or without 10μM LRH-1 peptide and cocultured with MiHA-specific CTL RP1 at a stimulation ratio of 1:1. PD-L knockdown resulted in < 20% PD-L1+ and/or < 1% PD-L2+ DC. (A) After 4 days, proliferation was determined following overnight [3H]-thymidine incorporation. Data of 1 of 2 independent experiments are shown, and bars are depicted as mean ± SD. CPM = counts per minute. (B) IFN-γ and (C) granzyme B levels were measured in CTL RP1 supernatant obtained at day 1 of coculture. Statistical analysis was performed using one-way ANOVA, followed by a Bonferroni post-hoc test. *P < .05, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-04-278739/6/m_zh89991060020004.jpeg?Expires=1769407954&Signature=PWGPM5ns0YANFMgYhItvoaFwdUWDM0EhsazlIFoidPSnIEwaW1fKZKx2MXc0VQCJbnyWf2pKBrKnAqkl9gI-R9XJbMdQrvKEmgfOuaaUWP0mELd3quRVmaKH70KyqIlHKBGNTIJpNss3NH2bZzXOwK~5rtMxY5O0Oc4k2NHuO~Tj4btdpXIF2yytN2-Wj4m72dRCf6r-qZ4ut9AakhY4won7uzL9zslN~V698tA4LgtNWrauLoGJ6L5cN785wjAM5Mc7QvzjeSaeM7b~MAdeW3Ztp4KNLSoPyhkWvPpyn5z40scBJEYfDRf84B7AT3NhKoQ9aKFTFQmOvlvgN7-89g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)