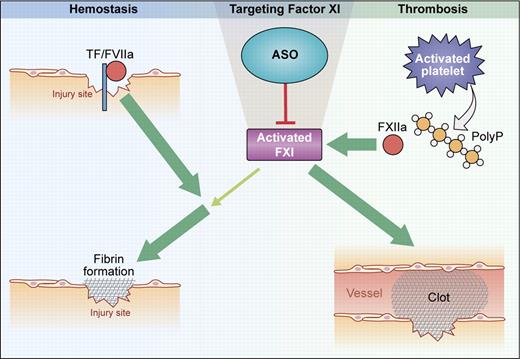
Function of FXI in hemostasis and thrombosis. Hemostasis: At sites of injury, fibrin formation is initiated by the tissue factor (TF)/factor VIIa complex. FXI is activated by thrombin and contributes to sustained fibrin production when TF activity is reduced by reaction with tissue-factor pathway inhibitor (TFPI). Thrombosis: On activated platelet surfaces, released polyphosphates (polyP) initiate contact activation of FXII that, in turn, activates FXI. Further FXI activity is generated by feedback activation. Targeting FXI with ASO abolishes pathologic thrombosis but has minor impact on hemostasis. (Professional illustration by Kenneth X. Probst.)
Function of FXI in hemostasis and thrombosis. Hemostasis: At sites of injury, fibrin formation is initiated by the tissue factor (TF)/factor VIIa complex. FXI is activated by thrombin and contributes to sustained fibrin production when TF activity is reduced by reaction with tissue-factor pathway inhibitor (TFPI). Thrombosis: On activated platelet surfaces, released polyphosphates (polyP) initiate contact activation of FXII that, in turn, activates FXI. Further FXI activity is generated by feedback activation. Targeting FXI with ASO abolishes pathologic thrombosis but has minor impact on hemostasis. (Professional illustration by Kenneth X. Probst.)
Coagulation is a complex process vital to hemostasis—the cessation of blood loss from an injured vessel—but, under pathologic conditions, otherwise life-saving mechanisms can precipitate life-threatening occlusive thrombotic events, collectively the most common causes of disability and death in the developed world. A major goal in anticoagulation therapy is to identify targets for blocking thrombosis without increasing the risk of dangerous bleeding; unfortunately, anticoagulant drugs currently in use (such as heparins, vitamin K antagonists, or direct inhibitors of factor Xa and thrombin) target molecules that are also essential for hemostasis. Therefore, therapeutic and prophylactic use of anticoagulant agents for thromboprotection is associated with potentially severe and fatal bleeding complications.
In the classical cascade model, fibrin formation is initiated by the intrinsic and extrinsic pathways of coagulation. The intrinsic pathway is activated by “contact” of factor XII (FXII, Hageman factor) to negatively charged surfaces in a reaction involving plasma kallikrein and high-molecular-weight kininogen (contact activation system). Activated FXII (FXIIa) triggers coagulation via activating its substrate, coagulation factor XI (FXI), that in turn contributes to fibrin formation by activating factor IX. Deficiency in factor IX results in severe hemorrhage in patients (hemophilia B), whereas FXI-deficient humans suffer from minor/relatively mild bleeding (hemophilia C), which is characterized by trauma or soft tissue–related hemorrhage, primarily involving tissues with high fibrinolytic activity. Bleeding tendencies vary substantially between patients with similar FXI plasma levels and are not directly related to FXI antigen levels. In contrast, FXII deficiency is not associated with any increased bleeding risk, indicating the existence of FXII-independent pathways for FXI activation.2 Thrombin has been shown to convert FXI to the active protease FXIa, and anti-FXI antibodies were found to interfere with sustained fibrin production by thrombin-driven FXI feedback activation in plasma.3
The role of FXI in hemostasis and thrombosis has been extensively studied in animal models.4,5 In contrast to patients with hereditary FXI deficiency, FXI-null mice do not bleed excessively when challenged by surgical procedures. FXI-null mice have not been systematically analyzed by injury to tissues with high fibrinolytic activity, so it is not known whether the protease is required for normal hemostasis in mice in some situations. Challenging the dogma of a coagulation balance, FXI-deficient mice have severely reduced thrombus formation in response to various types of mechanical and chemical vessel injuries in arterial and venous beds, although the animals have a normal hemostatic capacity. These mouse models show that FXI-mediated procoagulant activity is crucial for pathologic thrombosis.4 Interestingly, reconstitution of FXI-null mice with human FXI restores thrombus formation in the carotid artery in the ferric chloride injury model, suggesting that the protease operates similarly among the species.6 Indeed, the critical function of FXI for pathologic thrombosis has been convincingly demonstrated in other species. Targeting FXI in rabbits, rats, and baboons with inhibitory antibodies and peptidomimetic inhibitors potently reduced thrombus formation.
How is FXI activated in pathologic thrombosis? Initially, based on the normal hemostatic capacity of FXII-deficient humans, thrombin-mediated feedback activation was considered to be the major pathway for activating FXI in vivo.6 However, the discovery that vessel-occlusive thrombus formation both in FXII- and FXI-deficient mice was similarly defective suggests that FXIIa activates FXI in thrombosis.7 Indeed, we recently identified platelet-released polyphosphates, inorganic polymers of 80-120 orthophosphates, as the initiator of FXII-driven fibrin production in vivo; mice with combined deficiency in the intrinsic pathway proteases FXII and FXI (FXII−/−/FXI−/−) were protected from lethal polyphosphate-driven thrombosis to the same extent as the single gene-deficient mice, FXII or FXI nulls.8 Consistently, anti-FXI antibodies that specifically block FXIIa-mediated activation of FXI interfere with thrombus formation in vivo.9 Cumulatively, the mouse models reveal a crucial role of FXI for arterial and venous thrombosis and show that the protease is activated in a FXII-dependent manner—consistent with the classical reaction sequence of the intrinsic pathway.
Zhang and colleagues use antisense technology based on base-pair hybridization of antisense oligonucleotides (ASOs) with mRNA to inhibit FXI in mice (see figure). FXI-specific synthetic ASOs are internalized, translocate to the nucleus, and specifically hybridize with the complementary sequence of the FXI mRNA. The mRNA-ASO duplex is specifically degraded by nuclease RNase H and consecutive expression of the gene of interest is reduced. Intravenous infusion of FXI-specific ASOs dose-dependently reduced FXI plasma levels. When FXI was < 30% of normal, ASO-treated mice were largely protected from arterial and venous thrombosis in various models such as ferric chloride–induced thrombosis in the aorta, mesenteric veins, and the vena cava, as well as stasis-triggered vena cava thrombosis. Notably, thrombus formation in FXII and FXI heterozygous mice having 50% of normal plasma levels is indistinguishable from normal wild-type mice.7 Therefore, drugs that target expression of intrinsic pathway proteases need to substantially reduce FXII and/or FXI plasma levels to efficiently block thrombosis. Despite the striking thromboprotective effects, FXI-ASO treatment did not interfere with the hemostatic capacity of injected mice. Co-administration of FXI-ASO with the antiplatelet drug clopidogrel or heparin amplified the anticoagulant potency of these drugs but did not increase bleeding. Application of ASO for targeting FXI is a smart strategy. ASOs specifically interfere with FXI expression and do not affect levels of other coagulation factors. Antisense nucleotide treatment reversibly reduces FXI levels and the effect lasts for several days (plasma half-life of FXI is ∼ 2 days). A FXI concentrate is available for substitution therapy that could serve as antidote in case of unexpected bleeding or injury.
Is FXI-ASO therapy a potential strategy to interfere with thrombosis in humans? The answer is probably yes. Retrospective clinical studies analyzing thromboembolic disease in a population with severe FXI deficiency indicate that these individuals are protected from ischemic stroke and deep vein thrombosis but not from myocardial infarction.10 Antisense oligonucleotides are used in clinics and given that FXI-ASOs are functional in humans, the drug offers an exciting opportunity for a novel, effective, and safe(r) antithrombotic therapy with minimal therapy-associated bleeding risk.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■