This is a striking result for several reasons. First, the cohort included 73 patients receiving cells from HLA-mismatched donors, including 28 receiving haploidentical virus-specific cells in which one might reasonably expect that the risk of GVHD would be higher. In fact, there was no difference in the rate of GVHD in patients receiving virus-specific cells from matched and unmatched donors (both were low) despite the fact that the cells were lines rather than clones and were administered for the most part in the absence of immunosuppressive therapy. Concerns had been heightened earlier this year when Amir and colleagues demonstrated that 80% of virus-specific cell lines (including Epstein-Barr, cytomegalovirus [CMV], varicella zoster, and influenza-specific T cells) cross-reacted with allogeneic HLA molecules in vitro.2 Melenhorst and colleagues looked for this phenomenon in their own cell lines to be certain that the lack of GVHD that they observed clinically was not simply a fortunate association of nonreactive HLA pairings in donors and recipients or a particular feature of the way their virus-specific cells had been prepared. Instead, like Amir and colleagues, they found HLA alloreactivity of the virus-specific cells in vitro, including reactivity with HLA molecules expressed by the recipient of the cells in vivo.
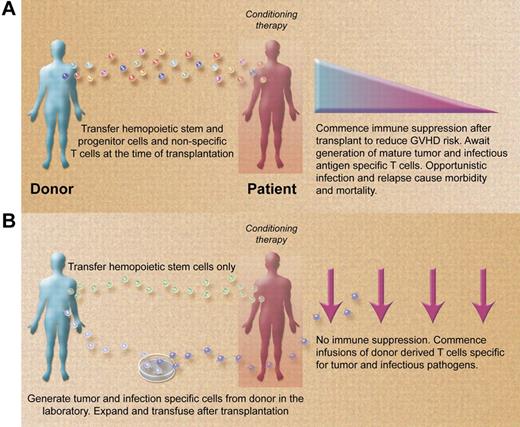
Paradigms for allogeneic stem cell transplantation (A) presently and (B) in the future. (A) Stem cell transplantations are performed with grafts containing stem and progenitor hemopoietic cells and nonspecific donor T cells. Posttransplant immune suppression required to control GVHD promotes opportunistic infection and disease relapse. (B) Stem cell transplantations are performed with grafts containing large numbers of purified stem cells depleted of nonspecific donor T cells. Posttransplant infusions of infection and malignancy specific T cells rapidly reconstitute the immune system in the absence of immune suppression, reducing the risk of opportunistic infection and disease recurrence. (Professional illustration by Alice Y. Chen.)
Paradigms for allogeneic stem cell transplantation (A) presently and (B) in the future. (A) Stem cell transplantations are performed with grafts containing stem and progenitor hemopoietic cells and nonspecific donor T cells. Posttransplant immune suppression required to control GVHD promotes opportunistic infection and disease relapse. (B) Stem cell transplantations are performed with grafts containing large numbers of purified stem cells depleted of nonspecific donor T cells. Posttransplant infusions of infection and malignancy specific T cells rapidly reconstitute the immune system in the absence of immune suppression, reducing the risk of opportunistic infection and disease recurrence. (Professional illustration by Alice Y. Chen.)
So why didn't more patients get GVHD when the virus-specific cells they were given reacted to a variety of allogeneic HLA molecules, including some of their own? The findings of Melenhorst and colleagues do not extend to providing the explanation, although some possibilities might include the relatively young age of the recipients (there was a weighting toward pediatric patients), the nature of their peritransplant T-cell depletion (many had received grafts depleted of T cells in vitro), the relatively small T-cell doses administered, and the memory cell as opposed to naive characteristics of the virus-specific cells. Whatever the case, the implication of their observation is clear. Virus-specific T cells, at least as prepared and administered by the groups in Washington and Houston, do not cause severe GVHD. Results from other groups using virus-specific T cells are also relevant. In an extension of a previously reported study,3 10 of 33 adults receiving bivirus-specific T cells after allogeneic transplantation in Sydney developed GVHD, de novo in 9 patients. Three patients developed grade III or IV GVHD. The group from London reported similar results with 11 of 30 patients developing acute GVHD after CMV-specific T-cell infusions with grade III disease in 3 of the patients.4 In both these series, GVHD rates were not different from those in similar cohorts not receiving virus-specific T cells. Randomized studies of both prophylactic and preemptive CMV-specific T cells currently running in the United Kingdom will provide further information on the risk of GVHD related to virus-specific T cells in patients receiving uniform conditioning.
In the meantime, the results of Melenhorst et al raise the prospect of developing an approach to allogeneic transplantation that combines reduction of GVHD using CD34+ or other purified hemopoietic stem cell grafts with rapid reconstitution of the immune system posttransplantation using specific T cells recognizing a broad range of infectious and malignant antigens (see figure). Such a transplant could theoretically be performed with little or no posttransplant immune suppression if the GVHD risk from T-cell infusions specific for other pathogens and malignant targets is as low as it appears to be for virus-specific T cells. Aspergillus-specific T cells have already been used clinically in the setting of haploidentical transplantation5 and several groups are studying the in vitro generation of T cells specific for other clinically relevant opportunistic pathogens including BK virus, varicella zoster virus, respiratory viruses, and others.
In addition to infections, cell therapy in the setting of T-cell depletion will need to deal with the issue of disease relapse. T-cell clones targeting minor histocompatibility antigens have been administered for relapsed leukemia in Seattle; although GVHD was observed, it did not appear to be worse than what existed before cell administration.6 Other cellular approaches to targeting malignant antigens include the production of cells bearing single-chain chimeric antigen receptors or artificial T-cell receptors recognizing relevant tumor antigens such as CD19 or WT-1.7–8 When used in the allogeneic transplant setting, every product will need to be assessed for both efficacy and GVHD risk. Each product targeting a specific infectious or malignant antigen that demonstrates efficacy with little or no GVHD risk will build one more brick in the wall of an immune system demolished by allogeneic stem cell transplantation. In coming years, we should welcome reports of new products that safely increase the spectrum of activity of cell therapy against specific antigens in the setting of allogeneic transplantation. More T anyone?
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal