Abstract
Abstract 4677
Acute Hemolytic Reactions (AHR) associated with administration of Rh(D) Immune Globulin (WinRho® SDF, Cangene Corporation, Winnipeg, MB, Canada) have been infrequently reported during the last 15 years. AHR's as defined were any case reporting hemoglobin decrease, haptoglobin decrease, elevation of bilirubin occurring within hours to number ofdays after WinRho® SDF treatment irrespective of the degree of change from baseline. AHR's where plasma or urinary hemoglobin were positive were categorized as cases of Intravascular Hemolysis (IVH).
To determine if communication tools such as publication of Case series (Gaines), Dear Doctor Letters and label changes stimulated reporting of AHR's and IVH.
Cangene's WinRho® SDF safety database from USA. was reviewed and analysed for the period 1995–2009. The reporting rate of AHR and IVH were calculated based on cases submitted by year and estimates of number of doses distributed in USA for ITP. These rates were trended to determine if the communication of safety risk was effective in stimulating reporting and decreasing rates of AHR or IVH cases.
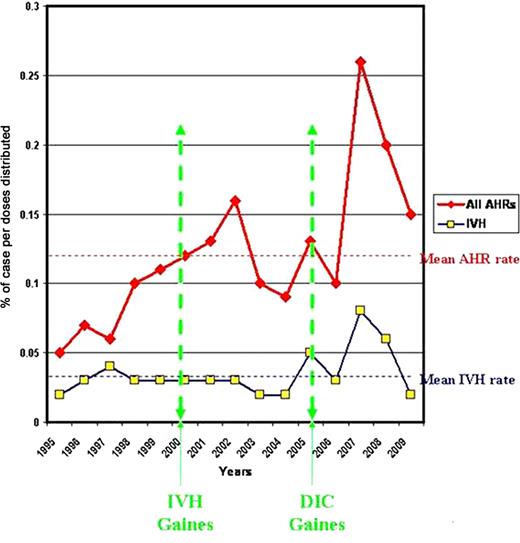
Gaines published 15 cases of IVH after WinRho® SDF in April 2000. Label changes and Dear Healthcare Professional (DHCP) letters were issued in 2000. Gaines reported 6 cases of disseminated intravascular coagulation with IVH in September 2005 and label was changed with Dear Healthcare Professional letters issued in Dec 2005. Reporting rate of IVH was not affected by these warnings. Increase in reporting rate of AHR's was seen after publications and warning letters in 2000 and 2005.
Yearly Reporting Rate of AHR and IVH
The overall reporting rate of AHR (including IVH) was estimated as infrequent (0.12%) while IVH reporting frequency was rare (0.03%).
Despite significant communication through FDA publications, medical education efforts and DHCP letters, there has been no appreciable change in reporting frequency of hemolytic events following Rh (D) Immune Globulin administration. This suggests that the initial estimate of IVH occurring at approximately 1:1000 doses (Gaines 2000) is still appropriate.
Guirgis:Cangene Corporation: Employment. Muller:Cangene Corporation: Employment. Little:Cangene Corporation: Employment. Sinclair:Cangene Corporation: Employment. Genereux:Cangene Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.