Abstract
Abstract 35
Patients with hematologic malignancies who are not in remission prior to allogeneic hematopoietic stem cell transplantation (HSCT) have a poor prognosis. In an effort to improve the anti-tumor activity of conditioning without added toxicity, we combined clofarabine, which is known to have a significant anti-leukemia activity, with myeloablative doses of busulfan in a phase I/II study in non-remission hematologic malignancies. Busulfan was administered as a single daily dose of 3.2 mg/kg IV × 4d (days -5 to -2) and clofarabine as a single daily dose of 20, 30 or 40 mg/m2 IV × 5d (days -6 to -2) with the specific dose determined by the Time to Event-Continuous Reassessment Method (TITE-CRM). All pts received dexamethasone 12 mg IV on the days of clofarabine to prevent capillary leak syndrome. Graft-versus-host-disease (GVHD) prophylaxis was tacrolimus/MMF in all but one patient (tacrolimus/methotrexate).
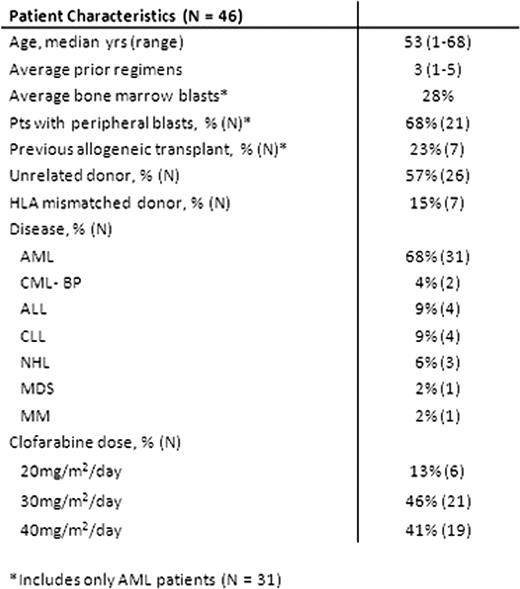
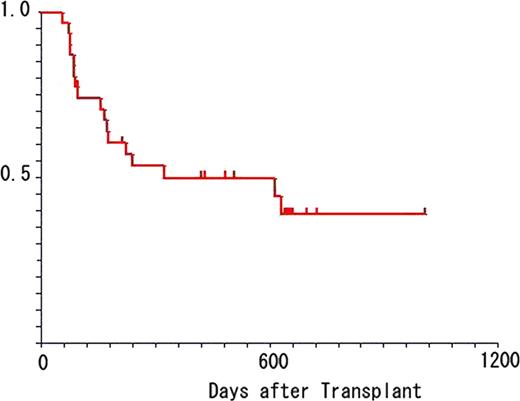
Forty six pts were enrolled. Characteristics of pts are shown in table 1. Prior to HSCT, pts failed an average of 3 regimens (range: 1–5), none were in remission, and 68% of leukemic pts had peripheral blasts. The majority received unrelated and / or HLA mismatched grafts. CloBu4 was generally well tolerated. Grade 3–4 non-hematological toxicities observed from initiation of conditioning to day +30 include: transient transaminitis (50%), mucositis (26%), hand-foot syndrome (13%), transient hypoxia (13%), nausea/vomiting (11%), diarrhea (11%), hypertension (7%), veno-occlusive disease (4%), hyperbilirubinemia (4%), hypersensitivity (2%), joint pain (2%) and seizure (2%). There were no cases of renal insufficiency and all cases of transaminitis resolved to ≤grade 1 within 14 days. All patients engrafted (median 11 days for neutrophils [range: 9–16]; 10 days for platelets [range: 1–20]. Lineage specific chimerism was analyzed at day 30, 100, 180, and 365. Full donor chimerism in CD3 lineage was achieved in 54%, 75%, 94% and 100% of pts on days 30, 100, 180 and 365, respectively. Absolute CD4 counts were 228±170, 238±147 307±151, and 341±184 cells/μ l on days 30, 100, 180, and 365, respectively. Acute GVHD (≥ grade 2) occurred in 48% of pts and resulted in five deaths. Overall, 80% of pts achieved CR by day +30 (AML = 94%, Others = 53%, AML without prior allo HSCT = 100%). Cumulative incidence of relapse in AML patients was 38%. The median duration of remission for AML pts was 15.4 months (range 2–34 m). Non-AML pts experienced a higher incidence of relapse/progression (67%, p=0.008) and shorter remissions (8.6 m, range 2–29 m). At a median follow up of 18 months, overall survival for the entire cohort was 42% at 18 months post-transplant (50% for AML, n=31). In conclusion, these data suggest that clofarabine combined with myeloablative doses of busulfan is well tolerated, facilitates engraftment, and has significant anti-tumor activity, particularly in patients with non-remission AML at HSCT. Given that CloBu4 reliably led to remissions that lasted a median of 15.4 months in non-remission AML pts, this regimen may provide a platform for further interventions such as maintenance therapy for this population of pts.
Mineishi:Genzyme: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding. Off Label Use: Clofarabine use for transplant conditioning regimen. Erba:Genzyme: Consultancy, Honoraria, Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.