Abstract
Abstract 281
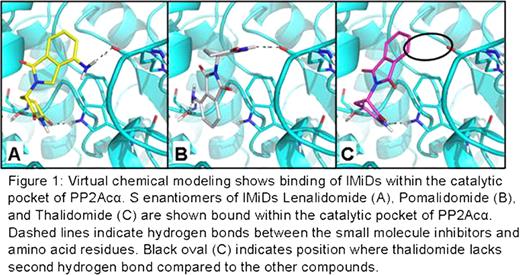
Lenalidomide generates high rates of transfusion independence and complete cytogenetic response in del(5q) MDS patients by selective clonal suppression. This family of immunomodulatory drugs (IMiDs) including lenalidomide (CC5013, Revlimid®) and pomalidomide (CC4047, Actimid®) are thalidomide analogues that also induce T cell receptor (TCR) co-stimulation, as well as augment NK and NKT cells by an unknown mechanism of action. Signaling by the T cell receptor (TCR) involves activation of a multi-subunit complex known as the Proximal Signalosome that orchestrates the ordered sequential events leading to phosphorylation of Src-family kinase members Lck and Fyn, phosphorylation of ZAP-70/Syk protein tyrosine kinases, recruitment of SLP-76, Grb-2, and Vav1. IMiDs operate through the B7-CD28 pathway, which provides co-stimulation and enhancement to the Signalosome pathway. To determine the molecular target, we evaluated the activation status of proteins involved in the TCR/CD28 Signalosome after lenalidomide treatment using a murine CD28-/- thymoma cell line transfected with full-length, wild type CD28 or a mutant (tailless) CD28 molecule lacking the intracellular signaling domain (Dennehy et al, 2007, J. of Immunology). We found that lenalidomide augments phosphorylation components downstream of CD28 in WT, but not in tailless mutant cells, suggesting that the intracytoplasmic domain of CD28 is necessary for drug response. We have previously shown that lenalidomide inhibits the activity of two haplodeficient phosphatases on chromosome 5q, Protein Phosphatase 2A (PP2A) and Cdc25c, located within the commonly deleted region (CDR). PP2Ac is known to bind to CD28 and is hypothesized to inhibit T cell co-stimulation. Therefore, it is plausible that lenalidomide and other IMiDs inhibit PP2A in T cells to augment proximal T cell signals. We examined this using molecular modeling and virtual screening. The application GLIDE (Schrödinger, L.L.C.) was used to estimate the free energy of binding for thalidomide, lenalidomide, and pomalidomide to the PP2A-ca heterodimer. The PP2A crystal structure (PDB 3K7V) was analyzed by SiteMap (Schrödinger, L.L.C.) to identify potential small-molecule binding sites. SiteMap identified the catalytic site which was then used for our binding simulations. The IMiD compounds and a known PP2A inhibitor, Fostriecin, were prepared for docking using LigPrep (Schrödinger, L.L.C.) which creates 3D geometry for the structures, provides alternative tautomers, ionization states (for pH values between 5.0 and 9.0), alternative ring conformations, and diastereomers. Free energies of binding and poses for the molecules suggest that both the R and S enantiomers of all three immunomodulatory drugs bind to the same position within the catalytic pocket of the c subunit of the PP2A heterodimer and thereby inhibit phosphatase activity. The S enantiomers of both lenalidomide and pomalidomide have better binding poses and energies (-5.80 kcal/mol and -6.06 kcal/mol respectively) than S thalidomide which has one hydrogen bond, instead of two, and a worse binding free energy than its enantiomer (-5.34 kcal/mol vs. -6.38 kcal/mol) (Figure 1). This increase in binding energy, when coupled with the change in binding pose suggests that S thalidomide would be an inferior inhibitor. The R enantiomers of all three drugs bind with nearly identical posing and binding free energy. Therefore, we hypothesized that, as a racemate, thalidomide may have reduced inhibitory potency compared to the other IMiDs. This was tested in vitro using hemaglutinin (HA)-tagged PP2A immunoprecipitated from stably transfected ad293 cells in a Malachite Green phosphatase assay. All of the drugs showed inhibition of phosphatase activity compared to control but interestingly, PP2A treated with 10μ M of lenalidomide and pomalidomide showed 40.4%±1.93 and 42.6%±0.29 decreased activity respectively compared with thalidomide (24.6%±7.5), which validated the modeling predictions. Together, these results suggest that IMiDs inhibit the enzymatic activity of PP2A, which augments TCR/CD28 Signalosome activity.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010