Abstract
Abstract 2463
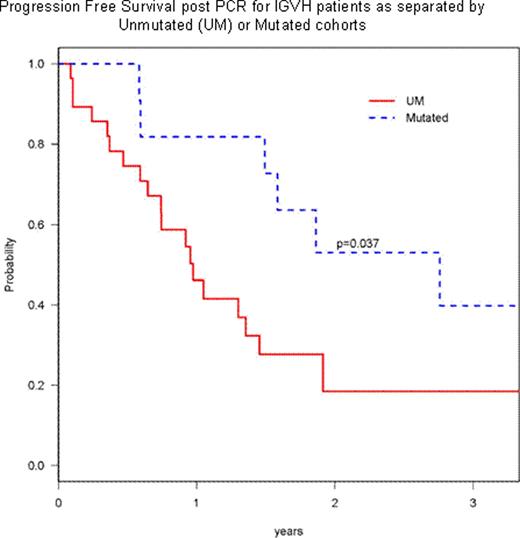
The treatment of relapsed/refractory B-Chronic Lymphocytic Leukemia (CLL) is difficult in terms of both inducing meaningful responses and being able to predict clinical outcome. In an attempt to address the latter aspect, we analyzed a battery of prognostic parameters obtained from a cohort of relapsed/refractory CLL patients entered onto ECOG trial E2903. This protocol included 2 steps: an induction phase (step 1) with pentostatin (4 mg/m2), cyclophosphamide (600 mg/m2), and rituximab (375 mg/m2) given every 3 weeks for 6 cycles and a consolidation phase (step 2) where complete responders (CR) were given 4 weeks of subcutaneous (sc) alemtuzumab (30 mg/m2, 3 times a week) or patients attaining partial response (PR), stable or progressive disease were given 18 weeks of sc alemtuzumab. Sixty-five eligible patients were enrolled; median age was 63 yr; male to female ratio of 4:1; median (range) number of prior therapies was 3 (1–7); and there were 16, 7, and 40 patients in Rai 0–1, Rai 2; and Rai 3–4 stages respectively. The median serum β-2 microglobulin was 4 (1–28). The overall response rate for step 1 in this cohort was 46% with 1 complete and 29 partial responses and 38.5% judged as having stable disease. Strict CR criteria, including CT imaging were utilized, demonstrating the infrequency of total response using CT scans. For traditional prognostic parameters, we found that age over 70 yr and high Rai risk stage (3–4) did not preclude a clinical response, but there was a significant difference between responders and non-responders in serum β-2 microglobulin levels (p=0.03). For molecular prognostic parameters, we found that the immunoglobulin variable heavy chain region (IGVH) mutational status did not preclude a lack of clinical response. However, for patients who were ZAP70 negative (−) or CD38 negative (−) these factors were positively associated with clinical response (p=0.046, 0.0005, respectively). The sensitivity in predicting a clinical response was 82% in ZAP70- and 73% in CD38-. In multivariable logistic regression analysis, only CD38 was significantly associated with response (Odds Ratio: 41, p=0.002). When patients were segregated into low (13q- or normal), intermediate (Trisomy 12) and high risk FISH (17p-,) there was no association with clinical outcome. In addition, if patients' CLL cells were unmutated and high risk FISH, there was no reduced clinical response. Of 65 patients included in this analysis, the 2-year overall survival (OS) was 55% (±7%) and the 2-year progression-free survival (PFS) is 25% (± 6.6%). The median follow-up time amongst survivors was 23 (range 6–60) months. The univariable analysis for PFS revealed that the IGVH mutated (2-yr PFS 53% for mutated vs. 18% for unmutated, p=0.037, Figure) and low risk FISH (2-yr PFS 42% for low risk vs. 23% for high risk, p=0.04) were significant. However, only IGVH mutated was significant (HR 0.33, p=0.027) for PFS in multivariable Cox model. In univariable analysis for OS, the combination of age <70 yr and β-2 microglobulin<2 upper limits [UL] was significant (2-yr OS: 68% vs. 39%, p=0.048), whereas only β-2 microglobulin was significant (HR 3.45 for >=2UL vs. <2UL, p=0.02) in multivariable Cox model. A total of 24 patients entered onto step 2 of the consolidation phase, receiving alemtuzumab; 17 patients who were responders from step 1 entered onto the 4 weeks of step 2; and 7 of the non-responders went onto the 18 weeks of step 2. The 2-year OS of these 24 patients was 62% from the date of consolidation phase: 61% for 17 responding patients to PCR and 60% for non responders to PCR (p=0.87). Current conclusions of this study are: (1) heavily pretreated high risk CLL patients may still have a respectable survival with the PCR combination; (2) β-2 microglobulin, ZAP70, and CD38 were important factors associated with response to PCR; (3) both IGVH mutated and FISH were prognostic markers associated with PFS; (4) addition of alemtuzumab may confer comparable survival rates for PCR non responders to the responders; (5) β-2 microglobulin was the only prognostic factor significant for OS. In total this study shows that both traditional and molecular markers can be used to predict clinical outcome for high risk relapsed/ refractory CLL patients treated with chemoimmunotherapy. We anticipate further valuable prognostic information to come from this still accruing study.
Progression Free Survival post PCR for IGVH patients as separated by Unmutated (UM) or Mutated cohorts
Progression Free Survival post PCR for IGVH patients as separated by Unmutated (UM) or Mutated cohorts
Rosen: Genentech: Speakers Bureau.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.