Abstract
Abstract 2223
Factor VIII (fVIII) inhibitory antibodies (fVIII inhibitors) are a significant source of morbidity in patients with hemophilia A. Approximately 30% of patients with severe hemophilia A will develop inhibitors. Most inhibitors are directed against either the A2 or C2 domains of fVIII. Anti-A2 antibodies have been reported to bind to at least 3 different regions of the A2 domain. Murine anti-human fVIII monoclonal antibody (MAb) MAb413 has an epitope that localizes to amino acids 484–508 and possibly blocks factor IXa binding to fVIII. R8B12 is a minimally inhibitory anti-A2 MAb that recognizes a discontinuous epitope that includes residues 497–510 and 584–593. CLB-Cag 9 is an anti-A2 MAb that recognizes amino acids 713–740 and decreases the rate of light chain cleavage by thrombin and factor Xa.
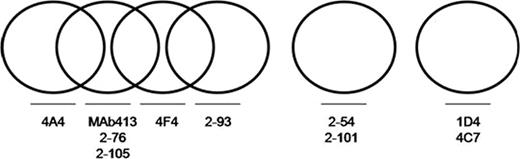
The goal of this study was to investigate the diversity of the humoral immune response to the A2 domain of human fVIII in a murine hemophilia A model. A panel of 10 murine anti-A2 antibodies was obtained, which included 9 MAbs from anti-fVIII hybridomas produced in our laboratory and antibody MAb413. All 10 MAbs were used as primary antibodies in a competition ELISA using human fVIII as the antigen. The same panel was biotinylated and used as secondary antibodies. Antibody pairs were classified as having non-overlapping or overlapping epitopes based on whether the binding of the secondary antibody was present or absent, respectively. The competition ELISA yielded 3 distinct groups of structural epitopes (see Figure). The ability of the MAbs to inhibit porcine/human hybrid fVIII proteins in a one-stage coagulation assay was used to further delineate the position of the structural epitope. Group 1 contained 6 antibodies, including MAb413, with 4 distinct epitopes on the fVIII protein. Group 2 contained 2 MAbs, and the epitope for 2–54 localized to amino acids 508–541. Group 3 contained 2 MAbs, and the epitope for 1D4 localized to amino acids 605–740.
Five of the 6 MAbs in Group 1 were Type I inhibitors with titers ranging from 330-40,000 Bethesda units (BU)/mg and one was non-inhibitory (see Table). In Group 2, both antibodies had high inhibitor titers (11,000 and 33,000 BU/mg) but were type II inhibitors with residual activities of ~20% at saturating concentration of antibody. Group 2 MAb 2–54 also was tested in a purified Xase assay and the residual fVIII activity was high (60-70%) at saturating concentration. Of the two MAbs in Group 3, one was non-inhibitory and the other was a Type I inhibitor with a titer of 7000 BU/mg. The concentration dependence of MAb413 (Group 1), 2–54 (Group 2), and 1D4 (Group 3) on fVIII cleavage and activity was tested by SDS-PAGE and the intrinsic Xase assay. With MAb413 a normal cleavage pattern was seen at concentrations that produced complete inhibition in the Xase assay. With 1D4, light chain, but not heavy chain, cleavage was inhibited in a concentration-dependent manner that corresponded to a decrease in Xase activity. This is consistent with CLB-Cag 9 which has a similar epitope at the C terminal end of the A2 domain. With 2–54, cleavage at all thrombin cleavage sites was partially inhibited, which corresponded to 30–40% inhibition in the Xase assay. This MAb represents a novel class of high titer, type II anti-A2 inhibitors that recognizes residues 508–541 and has similar in vitro characteristics to the Group BC anti-C2 MAbs. The elucidation of the structural and functional complexity of the anti-fVIII A2 repertoire should be useful in the characterization of the pathogenicity of A2 inhibitors.
A2 MAb Characteristics
| MAb . | Inhibitor Titer (BU/mg) . | Inhibitor Kinetics . | Group . | Structural Epitope . |
|---|---|---|---|---|
| 4A4 | 40,000 | Type I | 1a | |
| MAb413 | 21,000 | Type I | 1b | 484–508 |
| 2-76 | 38,000 | Type I | 1b | |
| 2-105 | 40,000 | Type I | 1b | |
| 4F4 | 330 | Type I | 1c | |
| 2-93 | <1 | n/a | 1d | |
| 2-54 | 33,000 | Type II | 2 | 508–541 |
| 2-101 | 11,000 | Type II | 2 | |
| 1D4 | 7000 | Type I | 3 | 605–740 |
| 4C7 | <1 | n/a | 3 |
| MAb . | Inhibitor Titer (BU/mg) . | Inhibitor Kinetics . | Group . | Structural Epitope . |
|---|---|---|---|---|
| 4A4 | 40,000 | Type I | 1a | |
| MAb413 | 21,000 | Type I | 1b | 484–508 |
| 2-76 | 38,000 | Type I | 1b | |
| 2-105 | 40,000 | Type I | 1b | |
| 4F4 | 330 | Type I | 1c | |
| 2-93 | <1 | n/a | 1d | |
| 2-54 | 33,000 | Type II | 2 | 508–541 |
| 2-101 | 11,000 | Type II | 2 | |
| 1D4 | 7000 | Type I | 3 | 605–740 |
| 4C7 | <1 | n/a | 3 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.