Abstract
Abstract 2085
Patients with metastasized breast cancer cannot be cured by current standard treatment options. One hypothesis is that slow cycling chemo-resistant tumor stem cells give rise to new tumors after cytoreductive treatment, ultimately leading to chemoresistant tumors. Last year we showed that the 4T1 mouse breast cancer model contains slow-cycling chemo-resistant cells that induce renewed growth of the tumor after chemo- and radiotherapy (abstract 4082). We also showed that haploidentical spleen and bone marrow transplantation (BMT) cures the mice and that donor NK cells are a prerequisite. Our current aim was to study the need of long term BM engraftment and to study the role of the conditioning in the curative process.
The 4T1 breast cancer cell line, originating from a spontaneous Balb/c (H-2d) breast cancer, was cultured under standard conditions. Fifty thousand 4T1 cells were injected s.c. in the flank. For the experiments addressing the need for haploidentical BMT tumor bearing CB6F1 (H-2b/d) recipients were treated with 2x 2Gy total body irradiation and 200 mg/kg cyclophosphamide (CY+TBI) followed by in vitro NK cell enriched haploidentical B6CBAF1 (H-2b/k) spleen cell infusion with or without additional BM cells. Chimerism in tumor-free surviving recipients was measured by flowcytometry of spleens at least 100 days after the treatment. The role of the conditioning in the alloreactive NK cell effect was studied in fully H-2 mismatched B6CBAF1 mice. When indicated, in vivo NK cell depletion was by i.p. injection of anti-AsialoGM1.
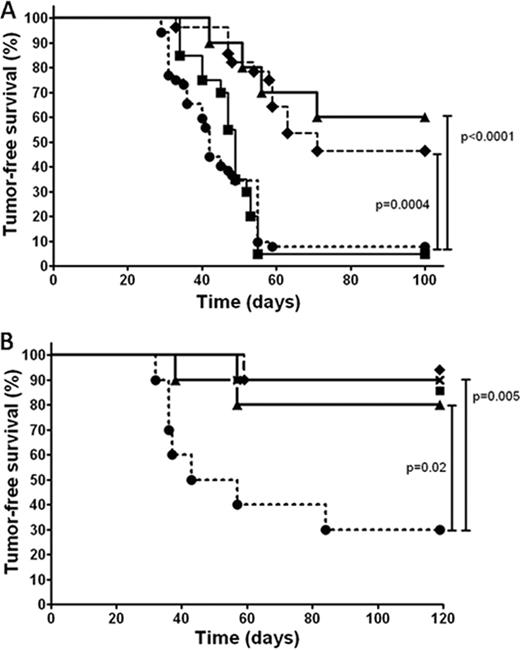
Figure A shows overall survival of mice with breast cancer after various treatments (10 mice per group). Haploidentical BMT plus spleen cells cured 50% of tumor bearing mice after CY+TBI (♦, dashed line) and survival was at least as good when NK cell enriched spleen cells were co-transplanted (▴, solid line). Transplantation of spleen cells from NK cell depleted mice (•, dotted line) obliterated the beneficial effect of haploidentical transplantation and resulted is similar poor survival as syngeneic BMT plus spleen cells (▪, solid line). The majority of mice that received NK cell enriched spleen cells (10 out of 14 tested) had no bone marrow engraftment and in the other four only 1–5% donor cells were detectable at 150 days. Recipients of unmanipulated haploidentical spleen and BM cells had >90% donor chimerism in 10 out of 14 tested. The cure rate in both groups was nevertheless similarly high. In a subsequent experiment (Figure B, 10 mice per group) we infused haploidentical NK cells only after CY+TBI (▴, solid line); other groups received T cell depleted (x, solid line) or T cell replete (♦, solid line) haploidentical BMT, or syngeneic BMT (▪, solid line). This resulted in a similar superior tumor-free survival (80-90%) than in mice co-transplanted with haploidentical BM (90%), as compared with syngeneic BM and spleen cell transplantation (•, dotted line).
We then planned to study the role of the conditioning in the curative process. For this purpose 4T1 breast cancer cells were injected in fully H-2 mismatched B6CBAF1 mice (H-2b/k). Surprisingly, 4T1 breast cancer is not rejected by B6CBAF1 mice despite the full MHC mismatch. Tumors are only rejected when the mice were treated with CY+TBI. Tumor rejection proved to be NK cell dependant and not a direct result of the conditioning as it was prevented by in vivo NK cell depletion.
This report provides the first evidence that chemo resistant tumor cells can be eliminated in vivo by alloreactive NK cells resulting in cure without the need for long term donor bone marrow engraftment. Conditioning with CY+TBI seems essential for this effect. These results set the stage for the exploration of alloreactive NK therapy in patients with metastasized breast cancer.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.