Abstract
Abstract 1189
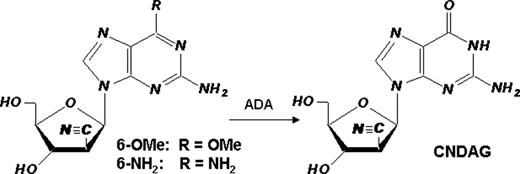
We hypothesize that the novel deoxyguanosine analogue CNDAG [9-(2-C-cyano-2-deoxy-1-β-D-arabino-pentofuranosyl) guanine] may share the common action mechanism with its cytosine congener CNDAC [2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine], a prodrug of which, sapacitabine, is undergoing clinical trials in myeloid leukemias. CNDAC induces single strand breaks following incorporation into DNA. Subsequent processing or DNA replication across the unrepaired nicks would generate double strand breaks (DSBs) [1]. Because cytosine and guanine nucleoside congeners have remarkably different clinical activities, e.g., cytarabine (acute myelogenous leukemia) and nelarabine (T-cell malignancies), it will be useful to pursue investigations to fully characterize the metabolism and actions of CNDAG. This study was aimed at defining cellular response and damage repair mechanisms for two CNDAG prodrugs, 2-amino-9-(2-C-cyano-2-deoxy-1-β-D-arabino-pentofuranosyl)-6-methoxy purine (6-OMe) and 9-(2-C-cyano-2-deoxy-1-β-D-arabino-pentofuranosyl)-2,6-diaminopurine (6-NH2). Each prodrug is a substrate for adenosine deaminase (ADA), the action of which generates CNDAG. First, growth inhibition by both CNDAG prodrugs was dependent upon both concentration and time of exposure; the proliferation of T-cell malignant lines (CCRF-CEM and Jurkat) was suppressed more to B-cell lines (Raji and IM-9). This may be attributed to relatively low activity of deoxycytidine kinase in the latter cell lines. Second, p53 knocked-out and parental HCT116 cells were equally sensitive to CNDAG 6-NH2 in a clonogenic assay, indicating that cytotoxicity of CNDAG is independent of p53 status. Third, similar to CNDAC, CNDAG prodrugs activated repair proteins in multiple DNA damage response pathways, as revealed by immunoblotting. 24-hr incubation of CCRF-CEM cells with 50 microM either prodrug increased the phosphorylation of Ser-1981 on ATM, Ser-345 on Chk1, Thr-68 on Chk2, Ser-966 on SMC1, Ser-343 on Nbs1 and g-H2AX. In contrast, there was no increase in phosphorylation of two other sensor kinases, DNA-PKcs (Ser-2056) which participates in repair of double strand breaks by non-homologous end-joining, and ATR (Ser-428) which senses stalled DNA replication forks. Fourth, we investigated the role of components of homologous recombination (HR) in CNDAG-induced DNA damage repair. The clonogenic survival of human fibroblasts deficient in ATM or those transfected with an empty vector were approximately 20- to 30-fold more sensitive to CNDAG prodrugs than cells complemented with full-length ATM cDNA. Chinese hamster cells deficient in Rad51D or either of the two Rad51-interacting proteins, Xrcc3 and Brca2, conferred greater than 30-fold sensitivity to CNDAG prodrugs relative to respective wild type lines. Similar sensitization was also observed with CNDAC. In contrast, cells lacking HR function were not more sensitive to ara-C or ara-G compared to their parental and complemented cells, indicating HR is a unique repair mechanism for 2`-C-cyano-2`-deoxy-nucleoside analogues. Finally, a cytogenetic approach was used to analyze sister chromatid exchange (SCE, a hallmark for HR) formation in metaphase cells exposed to 2 microM CNDAG 6-NH2. The frequencies of SCEs in AA8 cells incubated with CNDAG for two cell cycles (mean 14.2 per metaphase) were 2-fold of those exposed for one cell cycle (mean 7.4 per metaphase, n>20, p<0.001), the latter greater than control (mean 6 per metaphase, p<0.05). Together these results demonstrate that DNA damage caused by CNDAG activates ATM-dependent signaling pathways and is repaired through homologous recombination. Thus, this is a class effect caused by 2`-C-cyano-2`-deoxy-nucleoside analogues. Our study suggests that despite relatively less potency, CNDAG might have distinct clinical activity from that of CNDAC. [1] Liu X, et al. Blood, Blood. 2010 May 17, Epub ahead of print, PMID: 20479284.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.