Abstract
Purpose: To update American Society of Hematology/American Society of Clinical Oncology recommendations for use of erythropoiesis-stimulating agents (ESAs) in patients with cancer. Methods: An Update Committee reviewed data published between January 2007 and January 2010. MEDLINE and the Cochrane Library were searched. Results: The literature search yielded one new individual patient data analysis and four literature-based meta-analyses, two systematic reviews, and 13 publications reporting new results from randomized controlled trials not included in prior or new reviews. Recommendations: For patients undergoing myelosuppressive chemotherapy who have a hemoglobin (Hb) level less than 10 g/dL, the Update Committee recommends that clinicians discuss potential harms (eg, thromboembolism, shorter survival) and benefits (eg, decreased transfusions) of ESAs and compare these with potential harms (eg, serious infections, immune-mediated adverse reactions) and benefits (eg, rapid Hb improvement) of RBC transfusions. Individual preferences for assumed risk should contribute to shared decisions on managing chemotherapy-induced anemia. The Committee cautions against ESA use under other circumstances. If used, ESAs should be administered at the lowest dose possible and should increase Hb to the lowest concentration possible to avoid transfusions. Available evidence does not identify Hb levels ≥ 10 g/dL either as thresholds for initiating treatment or as targets for ESA therapy. Starting doses and dose modifications after response or nonresponse should follow US Food and Drug Administration–approved labeling. ESAs should be discontinued after 6 to 8 weeks in nonresponders. ESAs should be avoided in patients with cancer not receiving concurrent chemotherapy, except for those with lower risk myelodysplastic syndromes. Caution should be exercised when using ESAs with chemotherapeutic agents in diseases associated with increased risk of thromboembolic complications. Table 1 lists detailed recommendations.
Introduction
Use of erythropoiesis-stimulating agents (ESAs) has consistently been shown to reduce transfusions and increase the hemoglobin (Hb) level in patients with anemia that arises during or shortly after myelotoxic chemotherapy.1-7
The American Society of Hematology (ASH) and the American Society of Clinical Oncology (ASCO) first published a joint evidence-based clinical practice guideline for the use of epoetin in adults with chemotherapy-induced anemia in 2002.3 Since the 2002 guideline, awareness has grown of risks associated with ESAs, including increased mortality, venous thromboembolism, tumor progression, and stroke.8-25 Thus, specific guidance on the safe and optimal use of ESAs is warranted.
The initial guideline was updated and expanded in 2007 to include recommendations to address the use of darbepoetin alfa and emerging safety concerns.18 The current document is intended to update the 2007 guideline and examines the totality of data on ESA use, inclusive of data published since the 2007 guideline. It provides updated recommendations collectively for ESAs and reviews currently available information on ESA-associated tumor progression, venous thromboembolism, and/or survival, but does not revisit the effectiveness of ESAs to reduce transfusions or increase Hb in detail because the evidence on these outcomes is robust.
Guideline Questions
Two overarching questions clinicians are faced with when considering using ESAs are as follows.
What are the defining features of patients with a malignancy who are appropriate candidates for ESA treatment?
For patients who are appropriate candidates for treatment with ESAs, what are the optimal approaches to ESA therapy?
This guideline attempts to address these questions within the limitations of available evidence. Table 1 provides a summary of the guideline recommendations. The complete guideline, a patient guide, and other clinical tools and resources to help clinicians implement this guideline are available at http://www.hematology.org/guidelines/esa and www.asco.org/guidelines/esa.
Methodology
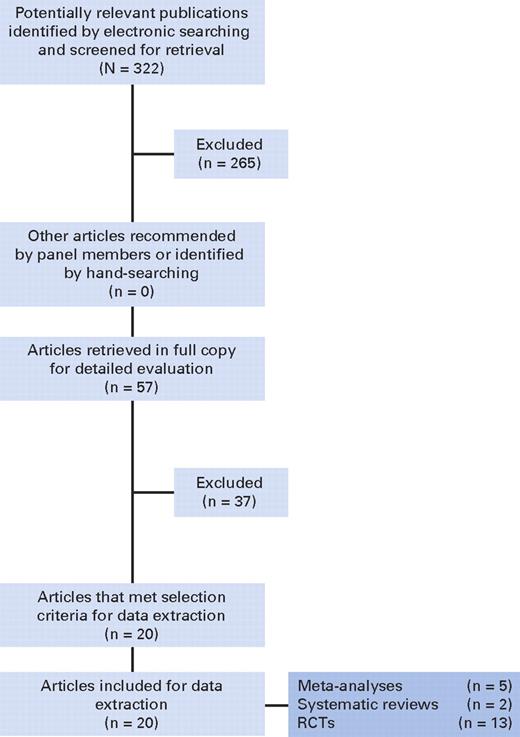
For the 2010 guideline update, the ASCO/ASH Update Committee completed a systematic review and analysis of data published since 2007. The Update Committee's literature review focused attention on available systematic reviews and meta-analyses of published phase III randomized controlled trials (RCTs) of ESAs. MEDLINE and the Cochrane Collaboration Library were searched from January 1 2007 to January 31 2010. The literature search strategy is available in the Appendix (online only). The literature search terms can be found in Data Supplement DS13. A summary of the literature search results is provided in a QUORUM diagram in Figure 1.
Exclusions and inclusions of publications identified for this systematic review. Literature search date parameter was January 1, 2007, to January 31, 2010 (inclusive). RCTs, randomized clinical trials.
Exclusions and inclusions of publications identified for this systematic review. Literature search date parameter was January 1, 2007, to January 31, 2010 (inclusive). RCTs, randomized clinical trials.
Panel Composition and Consensus Development Based on Evidence
The ASCO/ASH Update Committee was charged with reviewing evidence from the systematic review and making revisions to the guideline recommendations as warranted. The guideline was submitted to Journal of Clinical Oncology and Blood for peer review. The guideline was reviewed and approved by the entire Update Committee, ASCO's Clinical Practice Guidelines Committee, ASH's Committee on Practice, ASH's Subcommittee on Quality of Care, the ASCO Board of Directors, and the ASH Executive Committee.
Guideline Policy
The ASCO/ASH practice guidelines reflect expert consensus on the basis of clinical evidence and literature available at the time they are written and are intended to assist physicians in clinical decision making and identify questions and settings for further research. Because of the rapid flow of scientific information in oncology, new evidence may have emerged since the time a guideline was submitted for publication. Guidelines are not continually updated and may not reflect the most recent evidence. Guidelines address only the topics specifically identified in the guideline and are not applicable to interventions, diseases, or stages of disease not specifically identified. Guidelines cannot account for individual variation among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It is the responsibility of the treating physician or other health care provider, relying on independent experience and knowledge of the patient, to determine the best course of treatment for the patient. Accordingly, adherence to any guideline is voluntary, with the ultimate determination regarding its application to be made by the physician in light of each patient's individual circumstances and preferences. ASCO/ASH guidelines describe the use of procedures and therapies in clinical practice and cannot be assumed to apply to the use of these interventions in the context of clinical trials. ASCO and ASH assume no responsibility for any injury or damage to persons or property arising out of or related to any use of the ASCO/ASH guidelines or for any errors or omissions.
Guideline and Conflict of Interest
The ASCO/ASH Update Committee was assembled in accordance with ASCO's and ASH's respective conflict of interest policies. Members of the Update Committee were required to disclose financial and other interests that are relevant to the subject matter of the guideline, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as the result of promulgation of the guideline. Categories for disclosure include employment relationships, consulting arrangements, stock ownership, honoraria, research funding, and expert testimony. In accordance with the policies, the majority of the members of the Update Committee did not disclose any such relationships. For more information about the conflict of interest disclosures and policies, refer to the end of this article and to the unabridged version of the guideline (available at http://www.hematology.org/guidelines/esa and www.asco.org/guidelines/esa). The Update Committee membership is listed in Appendix Table A1.
Results
The literature search identified the following reports, published since the 2007 ASCO/ASH guideline update: one new individual patient data meta-analysis, six new literature-based meta-analyses and/or systematic reviews of RCTs, and 13 publications reporting RCT results not included in any of the meta-analyses or systematic reviews. Note also that since the 2007 update, the US Food and Drug Administration (FDA) and the companies who manufacture and/or market ESAs in the United States have created a Risk Evaluation and Mitigation Strategy (REMS). More detailed information is available online from the FDA.26
Meta-Analyses and/or Systematic Reviews of RCTs
Bohlius et al9,27 conducted a meta-analysis of survival outcomes using individual patient data from ESA trials. Six new literature-based meta-analyses and systematic reviews also met the inclusion criteria.8,20,28-34 The characteristics of these analyses are provided in Data Supplement DS1.
Several studies included in the new meta-analyses, available only as meeting abstracts or online in 2007, have now been published in peer-reviewed journals.35-39 Data from these studies were not extracted and are not discussed or summarized individually. A systematic review by Shehata et al20,32 also met the inclusion criteria but was not included in the data extraction because the review was essentially the same as an earlier online version20 included in the ASCO/ASH 2007 guideline update. These results are not discussed in detail in this update.
RCTs
Thirteen newly published articles reporting results from RCTs that met the inclusion criteria and were not included in any of the meta-analyses or systematic reviews are described in the Literature Update and Discussion sections in the recommendations, as appropriate.40-52 Characteristics of these trials are provided in Data Supplement DS2.
Primary Evidence Base for the Guideline Update
The individual patient data meta-analysis and other meta- analyses/systematic reviews serve as the primary evidence base for this guideline update. The consensus of the Update Committee was that, in general, all of these meta-analyses and systematic reviews are methodologically sound, although they differed in the totality of the trials and patients available at the time they were completed. Further comments about methodologic quality of individual meta-analyses and systematic reviews are provided in Data Supplement DS1. The Bohlius et al9,27 meta-analysis, for example, used individual patient data from all trials included in the analyses, whereas Glaspy et al33 used individual patient data from trials supported by two of the three manufacturers (a majority of the patients and trials they analyzed) and published aggregate data for remaining trials. For this reason, the Update Committee placed more weight in its deliberations on results from the Bohlius et al9,27 individual patient data meta-analysis. A summary of the data on the outcomes reported is provided in Data Supplements DS3-DS8.
2010 Guideline Recommendations
New evidence reported since the 2007 guideline update (summarized in Literature Update and Discussion for Recommendation I) establishes that, in addition to the previously demonstrated increases in thromboembolic event rates, ESA therapy is associated with shorter survival. However, evidence is still lacking on the mechanisms of these harms and, most importantly, on whether all patients are equally at risk or whether some patients may actually be at minimal risk for the harms associated with ESA use (with transfusion as necessary) compared with RBC transfusion alone. The Update Committee generally recommends that for patients undergoing myelotoxic chemotherapy who have Hb less than 10 g/dL, clinicians should discuss the potential harms (eg, thromboembolism, shorter survival) and benefits (eg, decreased transfusions) of ESAs and compare those with the potential harms (eg, serious infections, immune-mediated adverse reactions) and benefits (eg, rapid Hb improvement) of transfusion. Individual patient preferences for assumed risk should contribute to shared decisions on managing chemotherapy-induced anemia in these patients. The Update Committee cautions against ESA use under all other circumstances.
The guideline recommendations are summarized in Table 1, with substantive changes from the 2007 guideline noted in the table. The intended use of ESAs, as recommended in Table 1, is to reduce RBC transfusion requirements. All recommendations in the guideline are consistent with the FDA labels. The recommendations presented here provide further detail. The unabridged guideline update provides more comprehensive discussions and analyses of the systematic review and supportive evidence (available at http://www.hematology.org/guidelines/esa and www.asco.org/guidelines/esa).
I. General Recommendation
2010 recommendation.
It is recommended that before any decision regarding use of ESA is made, an appropriate history, physical examination, and diagnostic tests be conducted to identify alternative causes of anemia aside from chemotherapy or an underlying hematopoietic malignancy.
At a minimum, this would include:
Thorough drug exposure history
Review of a peripheral-blood smear (and in some cases, a bone marrow examination)
Analyses, where indicated, for iron, folate, or vitamin B12 deficiency
Assessment of reticulocyte count, occult blood loss and renal insufficiency
It may also include:
Coombs' testing for patients with chronic lymphocytic leukemia, non-Hodgkin's lymphoma, or a history of autoimmune disease
Assessment of endogenous erythropoietin levels for patients with myelodysplastic syndrome (MDS)
Consideration must be given to demonstrated risks of thromboembolism (see Recommendation IV), the possibility of death, and minimizing ESA use, particularly in patients with malignancy being treated with curative intent.
Special note.
Although the FDA label now limits the indication for ESA use to patients receiving chemotherapy for palliative intent, as described in Literature update and discussion: weighing harms versus benefits, no study has evaluated outcomes of ESA therapy by subgroups defined by chemotherapy intent. Determination of the goal of treatment requires clinical judgment in many cases.
Literature update and discussion.
As of the date of this publication, the FDA-approved labels state that the goal of ESA therapy for patients with chemotherapy-induced anemia is to reduce transfusion requirements. The only benefit of ESA therapy that has been unequivocally and consistently demonstrated in RCTs (including some that were placebo controlled and double blind) and meta-analyses is to reduce the need for transfusions as a result of increased Hb concentration (see Literature update and discussion: evidence on potential benefits). Transfusions will not be addressed in detail in this guideline update.
In rare circumstances, patients with cancer and renal insufficiency may have concurrent indications for the use of ESAs. Clinicians should also consider guidelines on ESA use for renal anemia under these circumstances (eg, National Kidney Foundation Disease Outcome Quality Initiative).53
In 2008, the FDA approved revised labels that limited the indication for ESA administration to patients receiving chemotherapy for palliative intent. ESAs are not indicated for patients receiving chemotherapy for curative intent. This change was made based on results of eight randomized trials and one meta-analysis available at that time, which suggested an increased risk of mortality with ESA use. Subsequent meta-analyses of RCTs and new data published from RCTs investigating differences in mortality with ESA use report similar findings.
Since the 2007 guideline update, one individual patient data meta-analysis,9,27 four literature-based or study-level meta-analyses,8,28,31,33,34 one systematic review of RCTs without a meta-analysis,29 and two individual placebo-controlled RCTs46,48 have published evidence relevant to the effects of ESA therapy on risk of mortality.
Literature update and discussion: new evidence on potential harms.
Bohlius et al9,27 conducted various meta-analyses of survival data (measured either over the study duration only or over all available follow-up) using individual patient data from 53 RCTs (pooled N = 13,933). ESA therapy was found to increase on-study mortality (hazard ratio [HR], 1.17; 95% CI, 1.06 to 1.30; P = .003) and mortality among patients in trials with chemotherapy (n = 10,441; HR, 1.10; 95% CI, 0.98 to 1.24; P = not significant; Data Supplement DS4). Estimation of the number needed to treat for an additional harmful outcome (in this case, the number of patients treated that would lead to one extra death) depends on the underlying survival probability in the absence of ESA treatment. For patients with an underlying survival probability of 95% at 1 year, the resulting estimate is that one additional person may die for every 121 participants randomly assigned to receive ESAs (number needed to harm [NNH], 121; 95% CI, 69 to 343), whereas the estimate based only on trials that included chemotherapy is that one additional person may die for every 206 participants randomly assigned to receive ESAs (NNH, 206; 95% CI, 86 to 1,026).27 If the underlying survival probability is 80%, the estimated NNH on the basis of all 53 trials would be 34 (95% CI, 19 to 94), and if the underlying survival probability is 70%, the estimated NNH would be 24 (95% CI, 14 to 67). For only trials that included chemo-therapy, if the underlying survival probability is 80%, the estimated NNH would be 57 (95% CI, 24 to 279), and if the under-lying survival probability is 70%, the estimated NNH would be 41 (95% CI, 17 to 200). ESA therapy also worsened survival over all available follow-up (HR, 1.06; 95% CI, 1.00 to 1.12; P = .046). There was no evidence for statistically significant heterogeneity across trials (I2 = 0%, P = .87 for on-study mortality, and I2 = 7.1%, P = .33 for survival over all available follow-up). A range of subgroups were also analyzed (eg, by patient characteristics, tumor characteristics, chemotherapy treatment) to determine whether there was any evidence of patient subgroups who were not at an increased risk of mortality. Meta-regression and statistical tests for interaction of potential modifying variables with the ESA treatment effect did not identify a set of factors that could be used to reliably select patients for whom the increased risk of mortality was minimal or negligible.
Findings from analyses of survival data in other meta-analyses and systematic reviews are provided in Data Supplement DS4. Findings from three literature-based meta-analyses8,28,31,34 are consistent with results of Bohlius et al,9,27 although the sample characteristics and study designs varied across these meta-analyses (Data Supplement DS1).
The random effects meta-analysis of overall survival in all patients with cancer reported by Glaspy et al33 yielded results (odds ratio [OR], 1.06; 95% CI, 0.97 to 1.15) similar to those reported by Bohlius et al27 (HR, 1.06; 95% CI, 1.00 to 1.12) but with wider CIs that did not reach the conventional level for statistical significance (P = .05). Results of random effects meta-analyses also were similar for trials in patients receiving chemotherapy (OR, 1.03; 95% CI, 0.93 to 1.13 reported by Glaspy et al33 v HR, 1.04; 95% CI, 0.97 to 1.11 reported by Bohlius et al27 ).
Several factors may contribute to the difference between the meta-analyses reported by Glaspy et al33 and Bohlius et al27 with respect to the statistical significance of increased mortality among patients randomly assigned to ESA treatment. See the unabridged guideline (available at http://www.hematology.org/guidelines/esa and www.asco.org/guidelines/esa) for detailed discussion of these differences and their potential impact on the findings. The Update Committee concluded that the methodologies used by Bohlius et al9,27 were more convincing than those used by Glaspy et al33 as evidence to support this recommendation.
None of the three new RCTs published since the 2007 guideline update demonstrated a statistically significant increased risk in mortality with ESA therapy compared with placebo.37,46,48 However, there were limitations with all three trials, and the P values likely reflect the small samples.
Evidence concerning thromboembolic risks associated with ESA use is summarized in the Literature Update and Discussion section of Recommendation IV.
Given the consistent results from the Bohlius et al9,27 individual patient meta-analyses and three of the four literature-based meta-analyses, the Update Committee placed more weight on these data than on the other reports (one literature-based meta-analysis, a systematic review, and three individual RCTs; Data Supplement DS4).
Literature update and discussion: evidence on potential benefits.
There is evidence from well-performed, randomized, placebo-controlled, double-blinded trials that ESA treatment decreases transfusion rates.19 This is the only benefit of ESA treatment that has been consistently demonstrated in RCTs and meta-analyses.
In 2006, the Agency for Healthcare Research and Quality (AHRQ) published a comparative effectiveness review of epoetin and darbepoetin.19 They found that the majority of trials reported fewer transfusions among patients randomly assigned to epoetin compared with patients assigned to control (RR, 0.63; 95% CI, 0.59 to 0.67).
Literature update and discussion: weighing harms versus benefits.
In the context of balancing the reported risks of ESAs against the reported benefits from using them as supportive care, the FDA-approved label was changed in August 2008 to state that use of ESAs is “not indicated for patients receiving myelosuppressive chemotherapy when the anticipated outcome is cure.”54 According to this product label, no studies have adequately characterized the impact of ESAs on progression-free and overall survival in the setting of curative-intent chemotherapy. The FDA-approved indication includes the option of ESA therapy, rather than transfusion support, as part of a palliative care chemotherapy regimen.
Unfortunately, as discussed in the Literature Update and Discussion sections in Recommendations I, IIIa, and IV, it cannot be determined from the available evidence whether any particular group of potential ESA recipients has a greater or lesser risk of harm than other patients with chemotherapy-induced anemia. The mechanisms of harm are also unclear. The FDA-approved label's distinction between patients being treated with curative versus palliative intent may assist clinicians as they compare and discuss with patients the risk-to-benefit ratios of an ESA versus RBC transfusions. However, it is worth reinforcing the point that the decision to limit the indication for ESAs to patients undergoing chemotherapy for palliation (treatment intent) is not on the basis of direct comparative analyses of data from clinical trials of ESA treatment. Available analyses of data from RCTs have not stratified results on the basis of the intent of any particular regimen used.
Note also that determining the goal of treatment requires clinical judgment. Examples of diseases for which the treatment goal should generally be considered curative include (among others) testicular cancer, first-line therapy of Hodgkin's disease, and early-stage solid tumors treated with adjuvant chemotherapy (eg, breast, colon, early lung).
The Update Committee acknowledges the FDA's assessment that the reported benefits of ESAs may be outweighed by risks considered unacceptable in patients who might otherwise expect cure from their chemotherapy. Clinicians are urged to exercise caution in considering ESA use in patients with malignancy being treated with curative intent. The Update Committee stresses the importance of including a detailed discussion between health care providers and their patients about the potential harms and benefits of ESA therapy.
II. Special Commentary on the Comparative Effectiveness of Epoetin and Darbepoetin
2010 recommendation.
This recommendation remains the same as in 2007. On the basis of a comprehensive systematic review comparing outcomes of epoetin and darbepoetin in patients with chemotherapy-induced anemia and on the basis of identical indications, warnings, and cautions in the relevant FDA-approved package inserts, the Update Committee considers these agents to be equivalent with respect to effectiveness and safety.
Literature update and discussion.
Since the 2007 guideline update, there have been no new studies that compared outcomes of epoetin and darbepoetin in patients with chemotherapy-induced anemia. Hence, there is no new evidence that would change the 2007 recommendation.
IIIa. Chemotherapy-Induced Anemia: Threshold for Initiating ESA Therapy
2010 recommendation.
The use of epoetin or darbepoetin is recommended as a treatment option that may be considered for patients with chemotherapy-associated anemia and an HB concentration that has decreased to less than 10 g/dL to decrease transfusions. RBC transfusion is also an option, depending on the severity of the anemia or clinical circumstances.
Literature update and discussion.
Systematic reviews that informed the 2002 guideline and 2007 update found insufficient evidence to conclude that initiating ESA therapy at Hb levels ≥ 10 g/dL either spared more patients from transfusions or decreased the number of RBC units transfused per patient when compared with starting therapy at Hb concentrations less than 10 g/dL. The literature search for the current update identified three articles published subsequently that reported on RCTs comparing immediate versus delayed initiation of ESA therapy,39,42,47 with one of these39 being a full publication of a meeting abstract that was included in the 2007 guideline update38 (Data Supplement DS9). There are several methodologic limitations to each of these RCTs, as well as differences between them, which are discussed in more detail in the unabridged guideline. The methodologic limitations and differences might have biased trial results and should be considered when comparing results across the trials. The differences in the RCTs make pooled analyses across them difficult to interpret.
Neither of the two new trials42,47 reported a statistically significant decrease in the proportion of patients who received transfusion in the arm randomly assigned to immediate ESA treatment compared with the arm randomly assigned to delayed treatment. Thromboembolic events were more frequent among patients treated immediately in both new trials,42,47 but neither trial reported test results for statistical significance of these differences (Data Supplement DS9).
Across all five available trials, the Hb threshold to begin ESA treatment varied. Hb threshold for treatment in the delayed arm was 9 g/dL in one trial,55 10 g/dL in three trials,39,47,56 and 11 g/dL in one trial.42
Although the Update Committee continues to find available evidence insufficient to conclude that initiating ESA therapy at Hb concentrations ≥ 10 g/dL decreases transfusion use relative to delaying treatment until Hb < 10 g/dL, the Committee also notes that available evidence does not demonstrate increased harms associated with starting ESA therapy at Hb concentrations ≥ 10 g/dL, compared with waiting until it decreases below that threshold. Furthermore, a post hoc reanalysis of data from a randomized trial comparing different dosing regimens of darbepoetin alfa suggests the question of Hb threshold for starting ESA therapy merits further investigation.49 Fewer transfusions (with nonoverlapping 95% CIs) occurred for the less than 10 g/dL stratum than for the ≥ 10 g/dL stratum in both arms of this study (Data Supplement DS11). Results also showed more frequent thromboembolic events but fewer on-study deaths in the ≥ 10 g/dL stratum than the less than 10g/dL stratum of each arm (CIs and statistical significance were not reported).
In the ASCO/ASH update of recommendations in 2007, the Committee advised that ESA therapy might begin as a patient's decreasing Hb concentration approached 10 g/dL. This advice was particularly relevant to patients facing multiple cycles of additional myelosuppressive chemotherapy and was based on the well-documented 2- to 6-week delay between the start of ESA administration and increases in the number of circulating mature RBCs (FDA labels for epoetin alfa [Procrit; Centocor Ortho Biotech, Horsham, PA] and darbepoetin alfa [Aranesp; Amgen, Thousand Oaks, CA]).57-59 However, evidence has emerged since the 2007 update showing that ESA administration is associated with a statistically significant increase in mortality risk (see Literature Update and Discussion section for Recommendation I in the unabridged guideline). In response to the new evidence, FDA-approved labeling for each ESA now states that, “Therapy should not be initiated at Hb levels ≥ 10 g/dL.” Furthermore, FDA recommends that dosing should be “titrated for each patient to achieve and maintain the lowest Hb level sufficient to avoid the need for blood transfusion.”
The Update Committee accepts that, although evidence is lacking to establish an optimally safe and beneficial Hb threshold for starting ESA therapy, it is clinically prudent in light of the new evidence to wait until Hb concentration decreases to less than 10 g/dL. Thus, the Committee has revised the recommended starting Hb concentration and treatment goal to reflect the current FDA-approved labels. However, the Committee acknowledges that rare clinical circumstances (such as severe pulmonary or cardiovascular comorbidities) may warrant careful consideration of ESA use when Hb levels are ≥ 10 g/dL.
IIIb. Chemotherapy-Induced Anemia: Initiating When Hb Is ≥ 10 g/dL but Less Than 12 g/dL
2010 recommendation.
An optimal level at which to initiate ESA therapy in patients with anemia with an Hb between 10 and 12 g/dL cannot be definitively determined from the available evidence. Under these circumstances, whether or not to initiate ESA treatment should be determined by clinical judgment, consideration of the risks and benefits of ESAs, and patient preferences (see Recommendations I and IV). RBC transfusion is an option when warranted by clinical conditions.
Literature update and discussion.
As discussed previously for Recommendation IIIa, conclusive evidence is lacking to show that beginning ESA therapy before Hb level decreases to less than 10 g/dL decreases transfusion use compared with waiting until Hb reaches that threshold. Evidence also is lacking to demonstrate that, for patients with either a specific combination of anemia symptoms or a particular level of severity of symptoms, ESA treatment outcomes are superior with immediate treatment.39,42,47,56 Similarly, available evidence does not identify specific concurrent illnesses to define patient subgroups in whom the benefits of starting ESA treatment before Hb decreases to less than 10 g/dL outweigh the risks. Note also that current FDA labeling for both epoetin alfa and darbepoetin alfa states that ESA use “should not be initiated at Hb levels ≥10 g/dL” and that it “has not been demonstrated in controlled clinical trials to improve symptoms of anemia, quality of life, fatigue, or patient well-being” (see FDA product labels for epoetin and darbepoetin).60,61
Literature update and discussion: ESAs and quality of life.
Although transfusion avoidance is generally the rationale for ESA treatment in patients with cancer, use of ESAs when the Hb is between 10 and 12 g/dL has been considered as a treatment on the basis of the hypothesis that it may improve quality of life (QOL) in patients with cancer.
Previous iterations of the ESA guideline emphasized that a substantially enhanced QOL related to reduced anemia after ESA use was a potential benefit that might justify use in some patients when the Hb was between 10 and 12 g/dL. Considerable research interest has centered on whether and by how much ESAs impact recipients' QOL. The rather modest QOL benefits reported to date must now be reconsidered in the context of more well-defined risks of death related to ESA therapy. These risks may also be related to intensity of ESA dosing in nonresponders (in this guideline and the unabridged guideline, see Special Commentary on ESAs, Tumor Response, and Survival and see Potential Mechanisms Mediating Tumor Progression and Increased Mortality), which may be particularly true for patients considered for ESA initiation in this Hb range on the basis of clinical circumstances.
Since the last guideline update, several clinical trials35,42,48 and one meta-analysis31 have included QOL as an end point for patients with chemotherapy-induced anemia randomly assigned to treatment with ESAs or placebo. The Update Committee does not find this evidence conclusive to show that ESA use, particularly at higher levels of Hb initiation, leads to substantial improvements in QOL (ie, perceived and valued by patients) as measured by the Functional Assessment of Cancer Therapy instruments,35,42 even when the Hb increases and transfusion decreases are statistically significant.
As discussed in the previous guideline update,18 assessment of QOL remains challenging. Reported studies continue to face methodologic limitations, as summarized in the unabridged guideline (available at http://www.hematology.org/guidelines/esa and www.asco.org/guidelines/esa).
Taken together with trials previously reported, treatment with ESAs in patients with chemotherapy-induced anemia leads to small, statistically significant increases in QOL. However, experts disagree on whether the magnitude of the effect size for the difference in QOL change scores (treated v control arms) observed in ESA RCTs meets the psychometric definition of a clinically meaningful change. Furthermore, any benefits with regard to improvements in QOL must now be considered in the context of increasing evidence of risks associated with ESA treatment in this population. The guideline Update Committee recommends that the goal of ESA use should be to avoid transfusions, as discussed in other sections of this update, without specific consideration of improvement in QOL as a target outcome.
Considering the evidence showing that ESA use is associated with a statistically significant increased risk of mortality and venous thromboembolism (see Literature Review and Discussion for Recommendations I and IV) and the inability to identify any patient subset with minimally increased risk using individual patient data meta-analysis,9 the Update Committee advises caution when considering ESA therapy in any patient whose Hb concentration is ≥ 10 g/dL. Decisions about ESA therapy should be based on clinical judgment of individual risks, benefits, treatment goals, and discussions with patients.
IV. Thromboembolic Risk
2010 recommendation.
This recommendation remains the same as in 2007. Clinicians should carefully weigh the risks of thromboembolism in patients for whom epoetin or darbepoetin is prescribed. RCTs and systematic reviews of available RCTs demonstrate an increased risk of thromboembolism in patients receiving epoetin or darbepoetin. Specific risk factors for thromboembolism have not been defined in these trials; therefore, clinicians should use caution and clinical judgment when considering use of these agents. Established, general risk factors for thromboembolic events include history of thromboses, surgery, and prolonged periods of immobilization or limited activity. Some diseases and treatment regimens have also been associated with higher risk of venous thromboembolic events (see Literature update and discussion).
Literature update and discussion.
Since the 2007 update, three literature-based meta-analyses have been published that evaluated the rates of thromboembolic events among patients treated with ESAs (Data Supplement DS6).31,33,34 All three meta-analyses reported a statistically significant increased risk for thromboembolic events in patients treated with ESAs. The risk was the same when the analysis was restricted to the 35 RCTs that evaluated ESA use and reported this outcome among patients who received chemotherapy (v anemia of cancer or radiotherapy trials). In a separate meta-analysis conducted by Glaspy et al33 on a subset of 18 chemotherapy RCTs that provided data over long-term (> 6 months) follow-up, the risk of venous thromboemboli was similar (6,498 patients; OR, 1.47; 95% CI, 1.24 to 1.74).
Results of five of six RCTs published subsequently are consistent with the meta-analyses, although none of the five trials conducted tests of statistical significance (Data Supplement DS11).36,37,46,48,49 However, one study (N = 120) reported no thromboembolic events of any grade in either arm.43
A fixed effects meta-analysis of 30 RCTs in the AHRQ comparative effectiveness review found that thromboembolic events were statistically significantly more likely to occur in patients administered epoetin compared with controls (RR, 1.69; 95% CI, 1.36 to 2.10; P < .001).19 The pooled event rates of thromboembolic events were 7% (range, 0% to 30%) in patients treated with epoetin versus 4% in controls (range, 0% to 23%), whereas the rates were 5% in patients treated with darbepoetin versus 3% in controls (only one darbepoetin trial reported thromboembolic event rates).
None of the more recent RCTs, systematic reviews, or meta-analyses provided estimates for NNH. The AHRQ review provides estimates, given individual baseline risk factors, for the number needed to treat to cause one additional thromboembolic event. Individual baseline risks of thromboembolic events were selected based on those reported in a review of known risk factors, such as tumor type, extent of cancer, treatment regimen, prior history of thrombosis, and presence of other risk factors such as surgery or immobilization.62 For patients with a baseline risk of 2.5%, one additional thromboembolic event is estimated to occur for every 58 patients treated with an ESA (95% CI, 36 to 111), compared with one thromboembolic event occurring for every 29 patients treated with a baseline risk of 5% (95% CI, 18 to 56), one event occurring for every 15 patients treated with a baseline risk of 10% (95% CI, nine to 28), and one event occurring for every seven patients treated with a baseline risk of 20% (95% CI, five to 14).
The meta-analyses and individual RCTs that have been published since the 2007 update demonstrate and consistently substantiate the increased risk of thromboemboli among patients receiving ESA therapy previously reported. The Update Committee continues to urge caution in the use of ESAs for patients judged to be at increased risk for thromboemboli. Patients with multiple myeloma who are being treated with thalidomide or lenalidomide and doxorubicin or corticosteroids are at particularly increased risk.63 There are no data from RCTs investigating concomitant use of anticoagulants or aspirin to lessen this risk.
V. Starting and Modifying Doses
2010 recommendation.
It is recommended that starting and modifying doses of ESAs follow FDA guidelines:
FDA-approved starting dose of epoetin is 150 U/kg three times a week or 40,000 U weekly subcutaneously.
FDA-approved starting dose of darbepoetin is 2.25 μg/kg weekly or 500 μg every 3 weeks subcutaneously.
Dose modification should follow FDA recommendations as outlined in Table 2.
Discontinue ESA treatment when chemotherapy concludes.
Evidence does not exist to support improved effectiveness or safety with alternative starting doses, dose schedules, or dose-modifying schedules.
Literature update and discussion.
The 2007 update concluded that evidence was lacking to demonstrate improved efficacy or safety using starting doses or dose modifications different from those in the FDA-approved labeling for epoetin alfa and darbepoetin alfa. Two trials were published subsequently that compared different ESA starting doses or modifications.44,52 The unabridged guideline and Data Supplements provide the specific details and results of these trials.
This recommendation remains unchanged because neither of the new studies provides evidence to conclude that outcomes of ESA therapy would be improved by use of an initial dose or dose-modification regimen other than those in the FDA-approved labels. Note that some aspects of the labels' starting dose and dose-modification recommendations have changed (Table 2).
VI. Discontinuing Therapy for No Response
2010 recommendation.
This recommendation remains the same as in 2007. Continuing epoetin or darbepoetin treatment beyond 6 to 8 weeks in the absence of response (eg, a < 1 to 2 g/dL increase in Hb or no diminution of transfusion requirements) does not seem to be beneficial, assuming an appropriate dose increase has been attempted in nonresponders as per the FDA-approved label, and ESA therapy should be discontinued. Patients who do not respond should be investigated for underlying tumor progression, iron deficiency, or other etiologies for anemia.
Literature update and discussion.
Since the 2007 guideline update, there have been no new studies that investigated indicators of response to ESAs. Hence, there is no new evidence that would change the 2007 recommendation.
VII. Hb Target
2010 recommendation.
Hb can be increased to the lowest concentration needed to avoid transfusions, which may vary by patient and condition.
Qualifying statement.
An optimal target Hb concentration cannot be definitively determined from the available literature. Modification to reduce the ESA dose is appropriate when Hb reaches a level sufficient to avoid transfusion or the increase exceeds 1 g/dL in any 2-week period to avoid excessive ESA exposure (see Recommendation V), considering the risks of ESAs (see Recommendation I). Specific dose-reduction recommendations are provided in Table 2.
Literature update and discussion.
The 2007 update summarized emerging (but at that time inconclusive) evidence suggesting that ESA therapy might increase the risk of mortality in anemic patients with cancer. Since then, individual patient data9 and literature-based8,31 meta-analyses of RCTs have shown statistically significant increases in the risk of mortality for patients randomly assigned to ESA treatment compared with controls. In the individual patient data meta-analysis, the effect of ESA treatment on risk of mortality was observed whether measured over the treatment period specified in each RCT's protocol or over the entire follow-up duration available. Of note, the individual patient data meta-analysis reported that in multivariate regression analyses, tests for interaction did not show statistically significant modification of the mortality increase by planned Hb ceiling (using subsets defined as < 13 v 13 to < 15 v ≥ 15 g/dL or using subsets defined by increments of 1 g/dL between 13 and 16 g/dL). Additionally, subset analyses by target Hb concentration (comparing subsets at < 13 v 13 to < 15 v ≥ 15 g/dL) found no statistically significant differences between subsets in mortality increase from ESA treatment (P = .98). Similarly, a literature-based meta-analysis31 reported that none of the variables tested (including the achieved Hb concentration and whether ESA treatment regimen adhered to the prior ASCO/ASH guideline recommendations) significantly moderated the association between ESA treatment and mortality. None of these analyses independently evaluated the dose-intensity of ESA treatment as a possible risk factor (see Special Commentary on ESAs, Tumor Response, and Survival). Thus, available data do not identify a target Hb concentration for ESA therapy (or its upper limit) that is entirely free from increased risk of mortality. The Update Committee revised this recommendation to reflect current FDA-approved labeling advice to use ESAs to avoid transfusions and for health care providers to avoid steep increases in Hb with ESA treatment.
VIII. Iron Monitoring and Supplementation
2010 recommendation.
This recommendation remains the same as in 2007. Baseline and periodic monitoring of iron, total iron-binding capacity, transferrin saturation, or ferritin levels and instituting iron repletion when indicated may help to reduce the need for ESAs, maximize symptomatic improvement for patients, and determine the reason for failure to respond adequately to ESA therapy. There is inadequate evidence to specify the optimal timing, periodicity, or testing regimen for such monitoring. Although iron replacement is generally recommended to augment response for ESA recipients with iron deficiency, there is inadequate evidence to consider the use of intravenous iron as a standard of care.
Literature update and discussion.
Since the 2007 update, three RCTs have been published that evaluated the effects of intravenous iron in combination with darbepoetin in patients with chemotherapy-induced anemia.41,45,51 Each of these trials report greater hematopoietic response and fewer transfusions in patients who received darbepoetin and intravenous iron compared with patients who received darbepoetin and oral or no iron supplementation (Data Supplement DS10).
Although the published studies suggest that use of intravenous iron may augment ESA response, study limitations (as outlined in the unabridged guideline) lead the Committee to recommend that currently available clinical evidence is insufficient to support intravenous iron as standard of care for adjuvant therapy in patients with cancer and anemia receiving recombinant erythropoietin therapy.
IX. Anemia in Patients Not Receiving Concurrent Chemotherapy
2010 recommendation.
It is recommended that ESAs not be used in treatment of anemia associated with malignancy in patients who are not receiving concurrent myelosuppressive chemotherapy. Use of ESAs in patients with lower risk MDS to avoid transfusions is an exception to this recommendation.
Literature update and discussion.
The substance of this recommendation has not changed from the 2007 update. The previous ASCO/ASH guidelines concluded that there was lack of conclusive evidence on outcomes of ESA therapy for patients not receiving chemotherapy.3,18 In March 2007, the FDA Oncologic Drugs Advisory Committee reviewed unpublished evidence of a statistically significant increase in mortality and no decrease in transfusion risk in the ESA arm of a large RCT for patients with mostly stage III or IV solid tumors not receiving concurrent chemotherapy. In response, the FDA issued a warning against ESA therapy for anemia in patients with cancer (solid tumors or nonmyeloid hematologic malignancies) who were not receiving concurrent chemotherapy. ESA manufacturers and the FDA also warned clinicians to discontinue ESA treatment when a patient's chemotherapy course was completed, and current FDA-approved labeling retains the same warnings.
ASCO/ASH's 2007 Update Committee also recommended against ESA treatment for patients with solid tumors not receiving chemotherapy, on the basis of data from the same large RCT, available on the FDA's Web site. Evidence reported since the 2007 update on outcomes of ESA therapy in patients with a malignancy not receiving concurrent chemotherapy is insufficient to change the 2007 recommendation.27,30,31,33,50 Specific details and results from the more recent evidence are provided in the unabridged guideline and Data Supplements. Although the analyses from Glaspy et al33 did not report statistically significant increases in mortality associated with ESA use, the 2010 Update Committee placed greater emphasis on the individual patient data analysis where results conflicted for several reasons, including availability of patient-level data for a greater proportion of studies and patients, use of meta-regression and formal tests of interactions terms, and use of a strict intent-to-treat analysis.
Glaspy et al33 performed a meta-analysis of the rates of thromboembolic events associated with ESA therapy in subsets of trials on chemotherapy-induced anemia versus trials on anemia of cancer and did not find statistically significant differences in the effects of ESAs on thromboembolic events in these two groups of patients.
The literature search did not identify any new RCTs published since the 2007 update and not included in any of the new systematic reviews or meta-analyses identified for this update that were limited to patients not receiving concurrent chemotherapy.
Literature update and discussion: patients with lower risk MDS.
The updated literature search identified one new RCT50 and a meta-analysis and systematic review of RCTs30 that reported outcomes of ESA therapy for patients with lower risk MDS. Two other meta-analyses identified in the literature search were excluded because they either pooled data from single-arm studies64 or analyzed studies comparing ESA use with versus without a granulocyte or granulocyte-macrophage colony-stimulating factor.65
X. Treatment of Anemia in Patients With Nonmyeloid Hematologic Malignancies Who Are Receiving Concurrent Chemotherapy
2010 recommendation.
This recommendation remains the same as in 2007. Physicians caring for patients with myeloma, non-Hodgkin's lymphoma, or chronic lymphocytic leukemia are advised to begin treatment with chemotherapy and/or corticosteroids and observe the hematologic outcomes achieved solely through tumor reduction before considering epoetin. If an increase in Hb is not observed after chemotherapy, treatment with epoetin or darbepoetin for patients with myeloma, non-Hodgkin's lymphoma, or chronic lymphocytic leukemia, who are being treated with palliative intent and who are experiencing chemotherapy-associated anemia, should follow Recommendations I through VIII. Particular caution should be exercised in the use of epoetin or darbepoetin concomitant with chemotherapeutic agents and diseases where risk of thromboembolic complications is increased (see Recommendation IV). Blood transfusion is also a therapeutic option.
Special note.
Although the FDA label now limits the indication for ESA use to patients receiving chemotherapy for palliative intent, as described in Literature update and discussion: weighing harms versus benefits, no study has evaluated outcomes of ESA therapy by subgroups defined by chemotherapy intent. Although patients with multiple myeloma and chronic lymphocytic leukemia often respond to first- or subsequent-line therapy, because these malignancies recur in most patients, determining the treatment intent requires clinical judgment of an individual patient's circumstances.
Literature update and discussion.
Since the 2007 guideline update, Bohlius et al9,27 published results from their individual patient data meta-analyses of mortality and survival data, which included subset analyses of patients with nonmyeloid hematologic malignancies who were receiving concurrent chemotherapy. Subset analyses, using individual patient data from patients with hema-tologic malignancies receiving chemotherapy, showed no signi-ficant differences in mortality over the active study period in patients in ESA arms compared with patients in control arms (HR, 1.12; 95% CI, 0.81 to 1.54) or in survival over all available follow-up for these patients (HR, 1.13; 95% CI, 0.96 to 1.33).9
Special Commentary on Esas, Tumor Response, And Survival
Since the publication of the 2007 guideline, the results of several randomized trials have either been published or become available in the public domain, with some additional studies reporting adverse health effects associated with ESA use in patients with cancer. In response, the labels for ESAs were revised by the manufacturers and approved by the FDA to alert physicians to shortened survival and/or increased risk of tumor progression or recurrence in eight RCTs involving patients with cancer of the head and neck, breast, or uterine cervix; non–small-cell lung cancer; or various lymphoproliferative malignancies or mixed nonmyeloid cancers.57-59
Six of these RCTs were abstracted in detail in the 2007 guideline update.13-16,22,68,69 The unabridged guideline provides a literature review of trials published since the 2007 guideline update that evaluated survival or tumor progression and summarizes evidence by cancer type (ie, head and neck, breast, lung, uterine cervix, and mixed nonmyeloid solid cancers).
Potential Mechanisms Mediating Tumor Progression and Increased Mortality
The association between ESA use and adverse outcomes in patients with anemia and cancer, which include an increase in mortality during the period of exposure and an increased risk of thromboembolic events,10,11 has led to restrictions on ESA use in this patient population. Given the clinical effectiveness of ESAs in alleviating anemia and reducing transfusion requirements in patients with anemia and cancer, understanding the mechanisms for the adverse effects of ESAs is essential to optimizing the benefits of these agents while avoiding their hazards. A detailed discussion of potential mechanisms mediating tumor progression and increased mortality, including the roles of cytokines and erythropoietin receptors, is provided in the unabridged guideline.
Patient Communication
Patient counseling regarding the risks and benefits of ESA therapy is essential to ensure that patients are making informed decisions. The Update Committee encourages health care providers to have an open dialogue with their patients to help them make informed decisions by considering the scientific evidence and weighing their individual risks with potential harms and benefits of ESA therapy.
In addition to providing a medication guide, health care providers should discuss the following with patients considering ESA therapy:
The goal of ESA therapy for patients with chemotherapy-induced anemia is to reduce RBC transfusion requirements.
The FDA has indicated that ESAs should not be given to patients who are being treated for cancer when the goal is to cure the patients (of cancer). There are potential harms and benefits of ESAs versus RBC transfusions, and patients may have specific risk factors.
ESAs have been found to shorten overall survival and/or speed tumor growth in some patients with cancer.
ESAs have risks of adverse events, such as blood clots, so individual risk factors need to be considered.
ESAs are not recommended for patients with cancer who are not receiving chemotherapy or who are receiving radiotherapy because ESAs have been associated with an increased risk of death in these patients.
Although there are some suggestions that ESA treatment may improve fatigue or QOL in some patients, the primary goal of ESA therapy should be to reduce transfusion requirements.
An acknowledgment form needs to be signed by patients to confirm that they have talked with their health care professional about the risks of ESAs.
The US FDA and the pharmaceutical companies that market ESAs in the United States have put in place an REMS to advise clinicians and to facilitate discussions with patients about the use of ESAs. The REMS requires health care professionals to provide a medication guide that explains the risks and benefits of ESAs to patients who receive ESAs. For more details, refer to the medication guide for epoetin alfa58 and the medication guide for darbepoetin alfa.57 Health care providers who prescribe ESAs to patients with cancer are also required to enroll in the Assisting Providers and Cancer Patients with Risk Information for the Safe Use of ESAs Oncology Program (APPRISE).26
Health Disparities
ASCO/ASH clinical practice guidelines represent expert recommendations derived from critical appraisal of the best available evidence relevant to prospectively formulated, well-focused clinical questions on optimal practices in management of oncologic diseases. However, racial, ethnic, and socioeconomic disparities in quality of health care exist and persist in the United States. Members of racial and ethnic minority groups and patients with fewer financial resources tend to have a higher burden of comorbid illness, are more likely to be uninsured or underinsured, face more challenges in accessing care, and are at greater risk of receiving care of poor quality than other Americans.70-74
Analysis of observational data from the Surveillance, Epidemiology, and End Results–Medicare database (on patients treated between 1991 and 2002 for colon, non–small-cell lung, or breast cancer or diffuse large B-cell lymphoma) suggests that, with respect to socioeconomic status, ESAs might have been used more frequently for patients above the median than for patients in the lowest quartile.75
It is possible that out-of-pocket costs of ESAs pose a barrier to patients with little or no prescription coverage or who are subject to cost-sharing strategies (ie, copayments). The guideline Update Committee encourages health care providers to include direct and indirect costs in their discussions with patients who are considering ESA therapy. ASH and ASCO support the development of resources to facilitate patient-provider communication about costs of cancer care.76
Estimated costs for darbepoetin and epoetin on the basis of Medicare Plan B average sales price, with no administration fees or other adjustments made, are provided in Data Supplement DS12. These prices were estimated from a third-party payor perspective, on the basis of reimbursement rates from the Centers for Medicare and Medicaid Services that are widely accepted by providers, computed at the manufacturer's average sales price. Other treatment-related direct and indirect costs were not considered, such as diagnostic laboratory tests. Actual treatment costs and reimbursement will vary considerably across regions, payors, institutions, and practices, as well as over time, and the reader should consult current local cost information specific to his or her practice setting.
The guideline Update Committee believes that patients with cancer should have equal access to ESAs, after consideration of their risks and benefits. However, current data do not help us understand whether differences in patterns of use reflect differences in access, whether disparities exist in patients' access to ESAs, or whether there are any particular groups who benefit more or less from their use. Awareness of possible disparities in quality of care and efforts to mitigate these disparities should be considered in the context of this clinical practice guideline.
Future Directions
There is clear evidence regarding the ability of ESAs to increase Hb and avoid transfusions. There is also evidence of harm associated with their use. Perhaps the most pressing need for additional research is studies that further clarify the mechanisms of harm and, particularly, the groups of patients or circumstances of clinical use that are least associated with these risks. This understanding is paramount to the ability of clinicians to extend the benefit of these drugs while reducing the risks.
Approved by the American Society of Clinical Oncology Board of Directors on July 7, 2010. Approved by the Executive Committee of the American Society of Hematology on July 14, 2010.
Editor's Note: This document represents an abridged version of the complete guideline update and contains updated recommendations with a brief discussion of the relevant literature. Readers should refer to the complete guideline update, which includes a comprehensive discussion and analysis of the literature and more evidence tables. The complete guideline is available at http://www.hematology.org/guidelines/esa and www.asco.org/guidelines/esa.
This guideline was developed through a collaboration between the American Society of Hematology and the American Society of Clinical Oncology and has been published jointly by invitation and consent in both Blood and Journal of Clinical Oncology. Copyright © 2010 American Society of Hematology and American Society of Clinical Oncology. All rights reserved. No part of this document may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without written permission by the American Society of Hematology or the American Society of Clinical Oncology.
Acknowledgment
The Update Committee is grateful to the American Society of Clinical Oncology (ASCO) Board of Directors, the ASCO Clinical Practice Guidelines Committee, the American Society of Hematology (ASH) Executive Committee, the ASH Subcommittee on Quality of Care, the ASH Committee on Practice, David P. Steensma, MD, and the reviewers for Journal of Clinical Oncology for their thoughtful reviews of earlier drafts. We also thank Carol Schwartz and Ann Alexis Prestrud for their assistance.
Authorship
Contribution: J.D.R. and M.B. conceived and designed the manuscript; P.H., J.S., and M.R.S. provided administrative support; J.D.R., M.B., P.H., J.S., and M.R.S collected and assembled data; and all authors analyzed and interpreted data, wrote the manuscript, and approved the final manuscript.
Conflict-of-interest disclosure: J.L.S. has served in a consultant or advisory role for Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Carol Schwartz, Government Relations and Practice Department, American Society of Hematology, 2021 L Street NW, Suite 900, Washington, DC 20036; e-mail: cschwartz@hematology.org.
Appendix
Literature search strategy
For the 2010 guideline update, pertinent published information was reviewed to address each of the guideline questions. As noted in the Results, one meta-analysis using individual patient data and six comprehensive systematic reviews and meta-analyses of randomized controlled trials (RCTs) served as the primary evidentiary basis for this update. An additional 13 papers met the inclusion criteria and reported results from RCTs that were not included in any of the meta-analyses or systematic reviews. Supplementary searches of the MEDLINE database (National Library of Medicine, Bethesda, MD) were conducted to identify relevant information (January 2007 through January 2010) from additional published RCTs, systematic reviews, meta-analyses, and practice guidelines for this update. A series of searches was conducted using the medical subject headings or text words “erythropoietin, recombinant,” “epoetin alfa,” “epoetin beta,” “darbepoetin alfa,” and “neoplasms,” and variants thereof. (Details of the searches can be obtained from guidelines@asco.org on request.) Search results were limited to human studies and English-language articles. Search terms can be found in Data Supplement DS13. Only trials that reported clinical outcomes in nonpediatric populations were included in the systematic review. Trials were excluded if they only reported hematologic response rates and/or Hb concentration. Publications were included if they reported retrospective analyses of previously pub-lished RCTs. Extraction and review of quality-of-life (QOL) data were limited to studies and systematic reviews that included a control arm not treated with an ESA, reported QOL results separately for each arm, and reported overall QOL scores (in addition to any subscale scores that may have been reported) from standardized, validated QOL instruments. Editorials, letters, and commentaries were excluded from consideration, as were systematic reviews and meta-analyses that were limited to single agents, on the basis of the US Food and Drug Administration's position that available ESAs are members of the same pharmacologic class. The Cochrane Library was searched for available systematic reviews and meta-analyses with the phrases “erythropoietin,” “epoetin,” “darbepoetin,” “cancer,” and “malignancies.” Directed searches based on the bibliographies of primary articles were also performed. Finally, Update Committee members and ASCO staff contributed articles from their personal collections.