Abstract
ATP, which has an important proinflam-matory action as danger signal, induces the semimaturation of dendritic cells (DCs) that can be associated with immune tolerance. We identified epidermal growth factor receptor ligands as target genes of ATPγS, a slowly hydrolyzed ATP derivative, by a gene profiling approach in DCs. Amphiregulin was the most highly up-regulated gene in response to ATPγS. Human monocyte–derived DCs and mouse bone marrow–derived DCs released amphiregulin (AREG) after purinergic receptor activation, with a contribution of P2Y11 and A2B receptor, respectively. Supernatants of LPS+ATPγS-stimulated DCs induced smooth muscle cell and Lewis Lung Carcinoma (LLC) cell growth in vitro. The coinjection of LPS+ATPγS-stimulated DCs or their supernatants with LLC cells increased tumor weight in mice compared with LPS-treated DCs. The preincubation of LPS+ATPγS-treated DC supernatants with an anti-AREG blocking antibody inhibited their positive effect on smooth muscle cell density and tumor growth. The present study demonstrates for the first time that DCs can be a source of AREG. ATP released from tumor cells might exert a tumorigenic action by stimulating the secretion of AREG from DCs. Antagonists of purinergic receptors expressed on DCs and anti-AREG blocking antibodies could have a therapeutic potential as antitumor agents.
Introduction
The interactions between the immune system and angiogenesis are determinant for the regulation of tumor growth. The mechanisms of these interactions are not well understood at this time. Dendritic cells (DCs) are known to play a central role in these interactions by releasing vascular endothelial growth factor-A (VEGF-A).1,2 VEGF-A is able to induce endothelial-like differentiation of tumor-infiltrating precursors of DCs and their migration to vessels to participate to vasculogenesis.3 VEGF-A was also reported to inhibit the function of mature DCs.4 Immune cells such as tumor-associated macrophages and T cells were also reported to regulate angiogenesis through the secretion of potent angiogenic mediators such as angiopoietin-2 and interleukin-17 (IL-17).5,6
We demonstrated previously that ATP up-regulates the expression by monocyte-derived DCs (MoDCs) of numerous genes that may play a role in immunosuppression, in particular thrombospondin-1 and indoleamine 2,3-dioxygenase.2,7 Some epidermal growth factor receptor (EGFR) ligands were also ATP target genes in MoDCs, in particular, amphiregulin (AREG), which was the most highly up-regulated gene. AREG is a cell type–dependent mitogenic factor that binds to ErbB1 receptor, also called EGFR.8 AREG is implicated in tumorigenesis and angiogenesis.9,10 Ma et al showed a reduction of the tumor mass and the intratumoral vascularization using an antisense cDNA of AREG in a breast cell line.11 A clinical study showed that there was a significant overexpression of AREG and VEGF in primary breast cancer.12 Kato et al showed that AREG can induce the proliferation of rat vascular smooth muscle cells and could be implicated in arterial remodeling.13
We demonstrated here for the first time that human and mouse DCs are a source of AREG, an EGFR ligand with tumorigenic properties.
Methods
Mice
Male 6- to 8-week–old C57BL/6 mice were obtained from Charles River Laboratories. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health (NIH) and were approved by the Comission d'Ethique et du Bien-Etre Animal (CEBEA) ethical committee.
Reagents
ATP, adenosine 5′-O-(3-thiotriphosphate; ATPγS), UTP, prostaglandin E2 (PGE2), adenosine (Ado), NECA (5′-(N-ethylcarboxamido)adenosine), suramin, MRS1754, and lipopolysaccharide (LPS) were obtained from Sigma-Aldrich. Monoclonal anti–human and anti–mouse AREG blocking antibodies were purchased at R&D Systems.
Preparation of human monocyte–derived DCs
Peripheral blood mononuclear cells were isolated from leukocyte-enriched buffy coats of healthy volunteer donors by standard density gradient centrifugation using Lymphoprep solution from Nycomed. Mononuclear cells (2.5 × 108) were allowed to adhere during 1 hour and 30 minutes at 37°C at 5% CO2 in 75-cm2 cell culture flasks. Nonadherent cells were removed, and adherent cells were cultured in 15 mL of culture medium (RPMI 1640 medium with 2mM l-glutamine, 25mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1mM sodium pyruvate, 20 μg/mL gentamycin, and 10% heat-inactivated fetal calf serum) supplemented with 800 U/mL granulocyte macrophage colony-stimulating factor (GM-CSF), and 500 U/mL of IL-4. GM-CSF and IL-4 were also added a second time 2 days after the adhesion step. Five days after the adhesion step, the purity of each cell preparation is evaluated by flow cytometry by analyzing the expression of 2 markers of DCs, human leukocyte antigen (HLA)–DR and CD1a. Moreover, the absence of monocytes, lymphocytes, and mature DCs was always checked by staining cell preparation using, respectively, CD14, CD3, and CD83 markers. For our experiments, we have only used cell preparations of HLA-DR+ CD1a+ immature DCs displaying at least 95% of purity.
Generation of DCs from mouse bone marrow progenitors
Bone marrow–derived DCs (BMDCs) were isolated as previously described with some modifications.14,15 Briefly, mouse bone marrow–derived progenitors were obtained from C57BL/6 mouse femora. Mouse bone marrow–derived progenitors (7.5 × 105 cells/plate) were cultured in 6-well plates in the presence of GM-CSF (20 ng/mL) for 9 days. After 3 days, an equal volume of fresh medium with GM-CSF was added per plate. At day 6, one-half of the culture medium was removed and replaced by fresh medium with GM-CSF. The purity of each cell preparation was checked by flow cytometry by analyzing the expression of CD11b, CD11c, CD86, and CD40 markers. For our experiments, we have only used cell preparations of CD11b+ CD11c+ immature BMDCs displaying at least 90% of purity.
Flow cytometric analysis
Human and mouse DCs were labeled with respective fluorochrome-conjugated monoclonal antibodies (PE-CD1a, CD14-PE, HLA-DR-PE, CD3-PE, CD83-FITC for human DCs; and CD11b-PE, CD11c-FITC, CD86-PE, CD40-PE for mouse DCs; all from BD PharMingen). Cells (2 × 105) were incubated in 100 μL of phosphate-buffered saline (PBS) with 0.1% sodium azide for 30 minutes in the dark at 4°C, washed with 1 mL of PBS, and analyzed on a Cytomics FC 500 (Beckman Coulter). Data were analyzed using CXP Version 2.0 cytometer software; the number of events was at least 10 000.
Quantitative RT-PCR experiments
Specific primers were selected for AREG, epiregulin, and heparin-binding EGF-like growth factor (HB-EGF) using Primer Express 2.0 software and the following criteria: polymerase chain reaction (PCR) product size, 100 to 150 base pairs; primer size, 20 to 25 base pairs; Tm, 58° to 60°C. Several control genes were tested for their stability in our system (YWHAZ, B2M, RPL13A, SDHA).16 Two of these control genes (B2M and SDHA) were selected after analysis using Genorm program. Reverse transcription (RT)–PCR amplification mixtures (25 μL) contained 2 n of template cDNA, Power SYBR Green PCR Master Mix (12.5 μL; Applied Biosystems) and 200 nM forward and reverse primer. Reactions were run on a 7500 Fast Real Time PCR System (Applied Biosystems). The cycling conditions were: 10 minutes for polymerase activation at 95°C and 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds. Mean ± SD were obtained for each gene using qBase Version 1.3.4 software. Each assay was performed in duplicate for 2 independent preparations.
Human and mouse AREG ELISA
Human and mouse DCs were stimulated by different agents for 24 hours at 106 cells/mL in 24-multiwells, and DC supernatants were collected. Human and mouse AREG level were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits from R&D Systems.
In vitro growth of primary HA-VSMCs
Human aorta vascular smooth muscle cells (HA-VSMCs) were incu-bated for 24 hours in 6-well plates at 7.5 × 104 cells/well in F12K medium supplemented with complements as described in ATCC data sheet (CRL-1999; ATCC). HA-VSMCs were incubated in low-serum medium (0.5% fetal bovine serum) for 24 hours. HA-VSMCs were then incubated for the next 48 hours with DC supernatants preincubated with or without an anti–human AREG blocking antibody during 3 hours. HA-VSMCs were then counted with hemocytometer.
In vitro growth of LLC cells
Lewis Lung Carcinoma (LLC) cells were placed for 48 hours in 12-well plates at 2 × 103 cells/well in Dulbecco modified Eagle medium supplemented as described in the ATCC data sheet (CRL-1642; ATCC). LLC cells were after incubated in low-serum medium (0.5% fetal bovine serum) for 24 hours. LLC cells were then incubated for the next 24 hours with BMDC supernatants preincubated with or without an anti–mouse AREG blocking antibody for 3 hours. LLC cells were then counted with a hemocytometer.
Mouse tumor model experiments
C57BL/6 mice were coinjected subcutaneously in left flank with LLC cells and with 5 × 105 BMDCs or 100 μL of BMDC supernatants preincubated or not with an anti–mouse AREG blocking antibody for 3 hours. Tumors were measured each 2 days starting at day 10, and tumor volume was calculated using the standard formula: A × B2 × 0.52, where A is the longest diameter, and B is the shortest diameter. Fourteen days after coinjection, mice were killed, tumors were extracted, weighted, embedded in Tissue-Tek OCT compound (VWR Scientific), and store at −80°C until cutting. Frozen tumors were cut into 7-μm thickness, fixed with methanol or 4% paraformaldehyde and stained overnight at 4°C with the following primary antibodies: anti-CD31 (rat, 1:400; BD Pharmingen), anti–αsmooth muscle actin (SMA)–Cy3 (mouse, 1:400; Sigma-Aldrich), anti-EGFR (rabbit, 1:400; Abcam), anti-CD11c (hamster, 1:400; BD Pharmingen), anti-AREG (rabbit, 1:600; Santa Cruz Biotechnology). Tumor sections were incubated for 1 hour at room temperature with appropriate fluorescent dye-conjugated secondary antibodies (Cy3/red and Alexa Fluor 488/green; Invitrogen), and finally, nuclei were counterstained with Hoechst 33342 (Invitrogen). Pictures of immunostaining were acquired at room temperature in FluorSave Reagent (Calbiochem) using an Axio Observer Z1 microscope, a high-resolution charge-coupled device camera, and Axiovision 4.6.3 software (Carl Zeiss).
Results
EGF-like growth factors up-regulation in response to ATPγS in human DCs
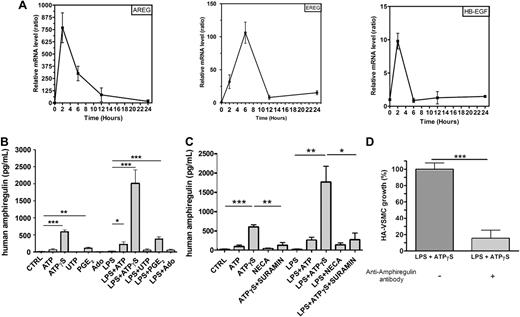
We have previously demonstrated that VEGF is a target gene of ATPγS in MoDCs using a microarray analysis.2 ATPγS is used because it is more resistant to degradation by ectonucleotidases than ATP. The microarray analysis also revealed that ATPγS up-regulates the expression of EGF-like growth factors such as amphiregulin, epiregulin, and HB-EGF genes. We have now confirmed the regulation of amphiregulin, epiregulin, and HB-EGF by ATPγS using quantitative RT-PCR (Figure 1A). MoDCs were stimulated with ATPγS for different periods of time: 2, 6, 12, and 24 hours. RNA was extracted and reverse-transcribed, and cDNA was used for SYBR Green real-time PCR (Figure 1A). The up-regulation of AREG mRNA in response to ATPγS was particularly strong and early (Figure 1A). Further studies were then focused on AREG.
Human monocyte-derived DCs secrete AREG. (A) Quantitative RT-PCR data for the EGFR ligands regulated by ATPγS. Ratios were obtained at 2, 6, 12, and 24 hours for 2 independent preparation of human MoDCs (means ± SEM) using SYBR Green technology. mRNA expression in ATPγS-treated cells and untreated cells has been normalized for each gene (AREG = amphiregulin, EREG = epiregulin, HB-EGF = heparin-binding EGF-like factor), and each time point using 2 housekeeping genes (B2M and SDHA). (B) AREG release by human MoDCs. DCs were stimulated by ATP (300μM), ATPγS (100μM), UTP (100μM), PGE2 (500nM), or Ado (10μM) in the absence or the presence of LPS (100 ng/mL) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of human AREG. Results represent the mean ± SEM of 6 independent experiments at least. The Student t test was performed using Prism 5.0 software (GraphPad; *P < .05; **P < .01; ***P < .001). (C) Effect of NECA and suramin on AREG release by human MoDCs. DCs were stimulated by ATP (300μM), ATPγS (100μM), NECA (1μM), or ATPγS (100μM) plus suramin (10μM) in the absence or the presence of LPS (100 ng/mL) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of human AREG. Results represent the mean ± SEM of 3 independent experiments. The Student t test was performed using Prism 5.0 software (*P < .05; **P < .01; ***P < .001). (D) HA-VSMC growth is increased by AREG secreted by MoDCs. DCs were stimulated by ATPγS (100μM) in presence of LPS (100 ng/mL) for 24 hours. Supernatants were treated or not with an anti–human AREG blocking antibody for 3 hours and incubated with HA-VSMCs for 48 hours. HA-VSMCs were then counted with hemocytometer. Data (mean ± SEM) are representative of 5 independent experiments. The Student t test was performed using Prism 5.0 software (***P < .001).
Human monocyte-derived DCs secrete AREG. (A) Quantitative RT-PCR data for the EGFR ligands regulated by ATPγS. Ratios were obtained at 2, 6, 12, and 24 hours for 2 independent preparation of human MoDCs (means ± SEM) using SYBR Green technology. mRNA expression in ATPγS-treated cells and untreated cells has been normalized for each gene (AREG = amphiregulin, EREG = epiregulin, HB-EGF = heparin-binding EGF-like factor), and each time point using 2 housekeeping genes (B2M and SDHA). (B) AREG release by human MoDCs. DCs were stimulated by ATP (300μM), ATPγS (100μM), UTP (100μM), PGE2 (500nM), or Ado (10μM) in the absence or the presence of LPS (100 ng/mL) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of human AREG. Results represent the mean ± SEM of 6 independent experiments at least. The Student t test was performed using Prism 5.0 software (GraphPad; *P < .05; **P < .01; ***P < .001). (C) Effect of NECA and suramin on AREG release by human MoDCs. DCs were stimulated by ATP (300μM), ATPγS (100μM), NECA (1μM), or ATPγS (100μM) plus suramin (10μM) in the absence or the presence of LPS (100 ng/mL) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of human AREG. Results represent the mean ± SEM of 3 independent experiments. The Student t test was performed using Prism 5.0 software (*P < .05; **P < .01; ***P < .001). (D) HA-VSMC growth is increased by AREG secreted by MoDCs. DCs were stimulated by ATPγS (100μM) in presence of LPS (100 ng/mL) for 24 hours. Supernatants were treated or not with an anti–human AREG blocking antibody for 3 hours and incubated with HA-VSMCs for 48 hours. HA-VSMCs were then counted with hemocytometer. Data (mean ± SEM) are representative of 5 independent experiments. The Student t test was performed using Prism 5.0 software (***P < .001).
Human MoDCs can secrete high amount of AREG
MoDCs were treated with ATP, ATPγS, UTP, PGE2, or Ado in the presence or the absence of LPS for 24 hours, and their supernatants were then collected to perform AREG ELISA (Figure 1B). ATP, ATPγS, and PGE2 induced a significant secretion of AREG by MoDCs that was potentiated by LPS, which had no effect alone (Figure 1B). We have then tested the effect of sura-min, which is known to antagonize P2Y11 receptor expressed on MoDCs (Figure 1C). We observed that ATPγS-mediated AREG secretion was inhibited by suramin (Figure 1C). NECA, which is a potent A1 and A2 agonist and more stable than adenosine, had a weak effect on AREG secretion in combination with LPS (Figure 1C).
AREG secreted by DCs increases smooth muscle cell growth
AREG is a mitogenic factor for many cell types expressing EGFR including vascular smooth muscle cells.13,17 To investigate the functional effect of AREG secreted by MoDCs, HA-VSMCs were incubated with DC supernatants pretreated or not with an anti-AREG blocking antibody (Figure 1D). Supernatants of DCs treated with the combination of ATPγS and LPS were used in these experiments because of their high content of AREG. Supernatants from DCs treated with LPS+ATPγS increased the number of HA-VSMCs, and this effect was abolished by the anti–human AREG blocking antibody (Figure 1D).
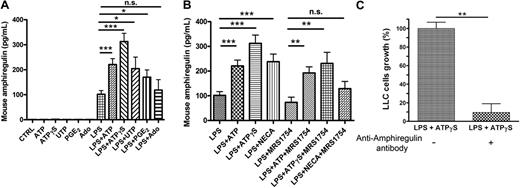
Mouse BMDCs can secrete AREG
To evaluate the tumorigenic properties of DC supernatants in vivo, we needed to investigate if mouse DCs were also able to secrete AREG. BMDCs were stimulated during 24 hours by ATP, ATPγS, UTP, PGE2, or Ado, in the presence or the absence of LPS. The tested agents were inactive in the absence of LPS (Figure 2A). We observed a significant AREG secretion in the supernatants of BMDCs treated with LPS+ATP or LPS+ATPγS (Figure 2A). The effect of UTP and PGE2 was more variable but significant, whereas adenosine had no effect (Figure 2A). We have also tested the effect of NECA and the potent A2B antagonist called MRS1754 on AREG secretion by BMDCs (Figure 2B). We observed a partial inhibition of ATP and ATPγS response by MRS1754, but there were still significant ATP and ATPγS effects in the presence of the A2B antagonist (Figure 2B).
Mouse AREG is released by BMDCs. (A) AREG release by murine BMDCs. BMDCs were stimulated by ATP (300μM), ATPγS (100μM), UTP (100μM), PGE2 (500nM), or Ado (10μM) in the absence or the presence of LPS (100 ng/mL) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of mouse AREG. Results represent the mean ± SEM of 6 independent experiments at least. The Student t test was performed using Prism 5.0 software (*P < .05; ***P < .001). (B) Effect of NECA and MRS1754 on AREG secretion by BMDCs. BMDCs were stimulated in the presence of LPS (100 ng/mL), by ATP (300μM), ATPγS (100μM), or NECA (1μM) in the absence or the presence of MRS1754 (4μM) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of mouse AREG. Results represent the mean ± SEM of at least 3 independent experiments. The Student t test was performed using Prism 5.0 software (**P < .01; ***P < .001; n.s., not significant). (C) LLC growth is increased by AREG secreted by BMDCs. BMDCs were stimulated by ATPγS (100μM) in presence of LPS (100 ng/mL) for 24 hours. Supernatants were treated or not with an anti–mouse AREG blocking antibody during 3 hours and incubated with LLC cells for 24 hours. LLC cells were then counted with hemocytometer. Data (mean ± SEM) are representative of 3 independent experiments. The Student t test was performed using Prism 5.0 software (**P < .01).
Mouse AREG is released by BMDCs. (A) AREG release by murine BMDCs. BMDCs were stimulated by ATP (300μM), ATPγS (100μM), UTP (100μM), PGE2 (500nM), or Ado (10μM) in the absence or the presence of LPS (100 ng/mL) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of mouse AREG. Results represent the mean ± SEM of 6 independent experiments at least. The Student t test was performed using Prism 5.0 software (*P < .05; ***P < .001). (B) Effect of NECA and MRS1754 on AREG secretion by BMDCs. BMDCs were stimulated in the presence of LPS (100 ng/mL), by ATP (300μM), ATPγS (100μM), or NECA (1μM) in the absence or the presence of MRS1754 (4μM) for 24 hours. Supernatants of treated DCs were collected for ELISA measurements of mouse AREG. Results represent the mean ± SEM of at least 3 independent experiments. The Student t test was performed using Prism 5.0 software (**P < .01; ***P < .001; n.s., not significant). (C) LLC growth is increased by AREG secreted by BMDCs. BMDCs were stimulated by ATPγS (100μM) in presence of LPS (100 ng/mL) for 24 hours. Supernatants were treated or not with an anti–mouse AREG blocking antibody during 3 hours and incubated with LLC cells for 24 hours. LLC cells were then counted with hemocytometer. Data (mean ± SEM) are representative of 3 independent experiments. The Student t test was performed using Prism 5.0 software (**P < .01).
The effect of DCs supernatants was then evaluated in the LLC tumor model. Supernatants from DCs treated with LPS+ATPγS increased the number of LLC cells after 24 hours; this effect was abolished when these supernatants were preincubated with an anti–mouse AREG blocking antibody (Figure 2C).
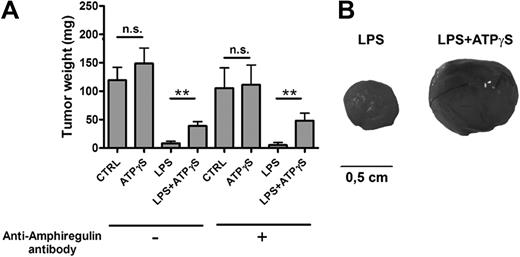
Coinjection of LLC cells and nucleotide-treated BMDCs increases tumor growth
BMDCs were treated for 24 hours with PBS (CTRL), ATPγS, LPS, or LPS+ATPγS and then treated or not with an anti–mouse AREG blocking antibody for 3 hours. LLC cells were then coinjected subcutaneously with 5 × 105 of these treated DCs in C57BL/6 mice. LLC tumors were weighted at day 14 (Figure 3A). The LPS effect on DC maturation led to an important inhibition of LLC tumor growth (Figure 3A). Such an inhibitory action of LPS on tumor growth, LLC in particular, has been reported previously.18,19 The action of ATPγS on BMDCs, in the presence of LPS, resulted in increased LLC tumor growth (Figure 3A-B). The preincubation of treated DCs with an anti–mouse AREG blocking antibody had no effect on LPS+ATPγS effect (Figure 3A).
BMDCs treated with LPS+ATPγS increased LLC tumor growth. (A) Coinjection of LLC cells and LPS+ATPγS-treated BMDCs increased tumor growth. BMDCs (5 × 105) were treated for 24 hours with PBS (CTRL), ATPγS (100μM), LPS (100 ng/mL), or LPS (100 ng/mL) plus ATPγS (100μM) and then incubated or not with an anti–mouse AREG blocking antibody for 3 hours. Treated BMDCs were coinjected with LLC cells (2.5 × 105), and LLC tumors were weighted at day 14. Results represent the mean ± SEM of 9 tumors at least for each condition. The Student t test was performed using Prism 5.0 software (*P < .05; **P < .01). (B) Pictures of LLC tumors 14 days after coinjection. Camera used to take pictures was a Nikon D70 with a Nikkor objective macro 60 mm.
BMDCs treated with LPS+ATPγS increased LLC tumor growth. (A) Coinjection of LLC cells and LPS+ATPγS-treated BMDCs increased tumor growth. BMDCs (5 × 105) were treated for 24 hours with PBS (CTRL), ATPγS (100μM), LPS (100 ng/mL), or LPS (100 ng/mL) plus ATPγS (100μM) and then incubated or not with an anti–mouse AREG blocking antibody for 3 hours. Treated BMDCs were coinjected with LLC cells (2.5 × 105), and LLC tumors were weighted at day 14. Results represent the mean ± SEM of 9 tumors at least for each condition. The Student t test was performed using Prism 5.0 software (*P < .05; **P < .01). (B) Pictures of LLC tumors 14 days after coinjection. Camera used to take pictures was a Nikon D70 with a Nikkor objective macro 60 mm.
AREG secreted by BMDCs treated with ATPγS plus LPS stimulates tumor growth in vivo
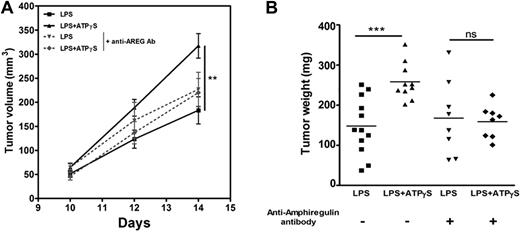
To focus on the potential role of AREG secretion on tumor growth, BMDCs were treated during 24 hours with LPS or LPS+ATPγS, and the supernatants (100 μL) were coinjected with 5 × 105 LLC cells. Tumor volumes were estimated after 10, 12, and 14 days (Figure 4A), and then the tumors were weighted (Figure 4B). We observed an increase of 74.6% ± 17.6% of tumor weight using supernatants of DCs treated with ATPγS+LPS com-pared with supernatants of DCs treated with LPS alone (mean ± SEM; ***; Figure 4B). The tumorigenic effect was abolished when the DC supernatants were preincubated with an anti–mouse AREG blocking antibody before coinjection with LLC cells (Figure 4B).
AREG secreted by BMDCs treated with ATPγS plus LPS promote LLC tumor growth. (A) Analysis of tumor volumes after coinjection of LLC cells and BMDC supernatants. BMDCs were treated with LPS or LPS+ATPγS during 24 hours, and their supernatants were incubated or not with an anti–mouse AREG blocking antibody for 3 hours. Tumor dimensions were measured at days 10, 12, and 14 after coinjection of LLC cells (5 × 105) and 100 μL of BMDC supernatants in C57BL/6 mice. Tumor volume was calculated using the standard formula: A × B2 × 0.52, where A is the longest diameter, and B is the shortest diameter. Results represent the mean ± SEM of 9 tumors at least for each condition. The Student t test was performed using Prism 5.0 software (**P < .01). (B) Effect of coinjection of LLC cells and BMDC supernatants on tumor weight. LLC tumor weight was measured at day 14 after coinjection of LLC (5 × 105) cells and BMDC supernatants. The Student t test was performed using Prism 5.0 software (***P < .001).
AREG secreted by BMDCs treated with ATPγS plus LPS promote LLC tumor growth. (A) Analysis of tumor volumes after coinjection of LLC cells and BMDC supernatants. BMDCs were treated with LPS or LPS+ATPγS during 24 hours, and their supernatants were incubated or not with an anti–mouse AREG blocking antibody for 3 hours. Tumor dimensions were measured at days 10, 12, and 14 after coinjection of LLC cells (5 × 105) and 100 μL of BMDC supernatants in C57BL/6 mice. Tumor volume was calculated using the standard formula: A × B2 × 0.52, where A is the longest diameter, and B is the shortest diameter. Results represent the mean ± SEM of 9 tumors at least for each condition. The Student t test was performed using Prism 5.0 software (**P < .01). (B) Effect of coinjection of LLC cells and BMDC supernatants on tumor weight. LLC tumor weight was measured at day 14 after coinjection of LLC (5 × 105) cells and BMDC supernatants. The Student t test was performed using Prism 5.0 software (***P < .001).
AREG increased the number of α-SMA–positive vessels in LLC tumors
LLC tumor sections were obtained from C57BL/6 mice coinjected with LLC cells and supernatants of BMDC treated with LPS or LPS+ATPγS. Sections were stained with Hoechst (blue) and with anti-CD31 (green) or anti–α-SMA (red) antibodies (Figure 5A). An increase of 30.7% ± 10.3% of α-SMA–positive cells was observed in tumors injected with supernatants of LPS+ATPγS-treated BMDCs compared with tumors injected with supernatants of LPS-treated BMDCs (mean ± SEM; n = 11; **), whereas CD31 staining was comparable. Quantification of vessels that are positive for both CD31 and α-SMA confirms a positive effect of supernatants of LPS+ATPγS-treated BMDCs (36.2% ± 10.8%: mean ± SEM; **), which was inhibited using the same DC supernatants preincubated with an anti–mouse AREG blocking antibody before injection (Figure 5B). Figure 5C shows the large expression of EGFR within the tumors. In particular EGFR expression was confirmed on α-SMA–positive cells, consistent with a direct proliferative effect of AREG on these cells.
AREG increased the number of α-SMA-positive vessels in LLC tumors. (A) CD31 and α-SMA staining of LLC tumors. LLC tumor sections were obtained from C57BL/6 mice coinjected with LLC cells and supernatants of BMDCs treated with LPS or LPS+ATPγS. Tumor sections were stained with Hoechst (blue) and with anti-CD31 (green) or anti–α-SMA (red). Arrows indicate CD31/α-SMA–double positive vessels (20×/0.4 numeric aperture [NA] objective). (B) Quantification of α-SMA and CD31–double positive vessels. Supernatants of BMDCs treated with LPS or LPS+ATPγS were incubated in the presence or the absence of an anti–mouse AREG blocking antibody. C57BL/6 mice were then coinjected with LLC cells and 100 μL of BMDC supernatants. At least 7 tumors (10 fields/tumor) were analyzed for each condition. The Student t test was performed using Prism 5.0 software (**P < .01). (C) SMA-positive cells express EGFR. Immunofluorescence analysis demonstrated colocalization of α-SMA expression (red) and EGFR (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows indicate α-SMA and EGFR–double positive cells (20×/0.4 NA objective).
AREG increased the number of α-SMA-positive vessels in LLC tumors. (A) CD31 and α-SMA staining of LLC tumors. LLC tumor sections were obtained from C57BL/6 mice coinjected with LLC cells and supernatants of BMDCs treated with LPS or LPS+ATPγS. Tumor sections were stained with Hoechst (blue) and with anti-CD31 (green) or anti–α-SMA (red). Arrows indicate CD31/α-SMA–double positive vessels (20×/0.4 numeric aperture [NA] objective). (B) Quantification of α-SMA and CD31–double positive vessels. Supernatants of BMDCs treated with LPS or LPS+ATPγS were incubated in the presence or the absence of an anti–mouse AREG blocking antibody. C57BL/6 mice were then coinjected with LLC cells and 100 μL of BMDC supernatants. At least 7 tumors (10 fields/tumor) were analyzed for each condition. The Student t test was performed using Prism 5.0 software (**P < .01). (C) SMA-positive cells express EGFR. Immunofluorescence analysis demonstrated colocalization of α-SMA expression (red) and EGFR (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows indicate α-SMA and EGFR–double positive cells (20×/0.4 NA objective).
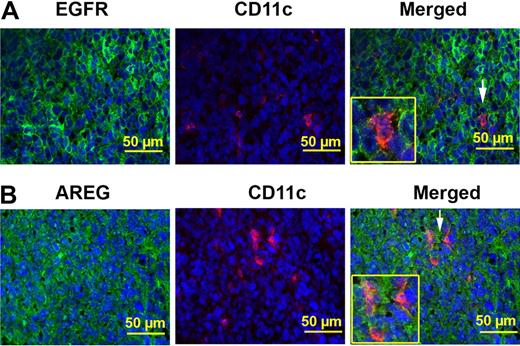
Tumor-associated DCs express AREG and EGFR
After coinjection of LLC cells and BMDCs or BMDC supernatants, AREG, and EGFR expression was evaluated in tumor-associated DCs by staining with anti-EGFR, anti-AREG, and anti-CD11c antibodies (Figure 6). We observed a large expres-sion of EGFR and AREG within the tumors. In particular, immunofluorescence experiments showed that CD11c-positive cells identified within the tumors express both EGFR and AREG (Figure 6A-B).
Tumor-associated DCs express AREG and EGFR. (A) Tumor-associated CD11c+ cells express EGFR. Immunofluorescence analysis demonstrated that cells stained with CD11c (red) are also positive for EGFR (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows point to CD11c and EGFR–double positive cells (40×/0.75 NA objective). (B) Tumor-associated CD11c+ cells express AREG. Immunofluorescence analysis demonstrated that cells stained with CD11c (red) are also positive for AREG (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows point to CD11c and AREG–double positive cells (40×/0.75 NA objective).
Tumor-associated DCs express AREG and EGFR. (A) Tumor-associated CD11c+ cells express EGFR. Immunofluorescence analysis demonstrated that cells stained with CD11c (red) are also positive for EGFR (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows point to CD11c and EGFR–double positive cells (40×/0.75 NA objective). (B) Tumor-associated CD11c+ cells express AREG. Immunofluorescence analysis demonstrated that cells stained with CD11c (red) are also positive for AREG (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows point to CD11c and AREG–double positive cells (40×/0.75 NA objective).
Discussion
Solid tumors contain both malignant cells and a variety of stromal cells, such as fibroblasts, endothelial cells, and inflammatory cells including T lymphocytes, macrophages, and DCs. It has been established that immune response dysfunctions in the tumor microenvironment could lead to tumor escape.20 Several studies have described the diversity of tumor escape strategies and notably, alterations in T-cell receptor signaling, suppression of T-cell responses by T regulators, suppression of natural killer activity, and decreased expression of costimulatory molecules.21 Furthermore, some studies have shown that immune cells can also contribute to promote tumor growth. Indeed, it has been described that tumor-associated macrophages stimulate tumor angiogenesis through VEGF secretion.22 Moreover, transforming growth factor-β, produced by tumor infiltrating T regulatory lymphocytes, is able to promote tumor progression.23
Many studies have shown that DCs can play a role in tumor escape from immunity.24-27 Indeed growing tumors contain DCs that are unable to induce antitumoral immune responses and, most importantly, that can induce T-cell tolerance.28,29 Furthermore DCs can promote tumor angiogenesis both by their secretion of proangiogenic cytokines and by their ability to transdifferentiate into endothelial-like cells.1,3,30,31
ATP is considered as a danger signal released from necrotic cells and in response to various forms of stress. Using a chimeric plasma membrane-targeted luciferase, Di Virgilio and colleagues have shown an increased level of extracellular ATP at tumor sites (hundreds micromolar range) compared with healthy tissues.32 The study of ATP actions on cells present in the tumor microenvironment is thus of major interest to understand the mechanisms of tumor growth. ATP is known to induce a semimaturation of DCs characterized by an increased expression of costimulatory molecules and a decreased secretion of IL-12.33,34 In human MoDCs, the P2Y11 receptor mediates ATP action occurring through cAMP increase and mostly reproduced by cAMP-elevating agents such as PGE2.34 Furthermore ATP has been shown to increase the expression by MoDCs of immunosuppressive proteins such as indoleamine 2,3-dioxygenase that could play a role in immune tolerance and of VEGF that could play a role in tumor angiogenesis.2,7
In this study we have demonstrated that AREG was the ATPγS target gene that was the most highly up-regulated in human DCs. Furthermore, we have also shown that human and mouse DCs are able to secrete high amounts of AREG in response to ATP and ATPγS, especially when combined with LPS. In BMDCs, ATPγS or ATP alone was not sufficient, and an inflammatory signal such as LPS was needed to initiate AREG secretion. It is important to note that AREG secretion requires the activation of a desintegrin and metalloprotease named ADAM17.35-37 The fact that AREG secretion by DCs was clearly stronger in response to stable ATPγS instead of ATP supports that a continuous release of ATP is needed in vivo because of its rapid degradation.
In vivo experiments were performed with treated-BMDCs or their supernatants to discriminate cell-dependent effects from the effect of released soluble factors. The protumoral effect of LPS+ATPγS-treated DCs coinjected with LLCs could be related to their tolerogenic features.7,33 We demonstrated that supernatants of LPS+ATPγS-treated DCs were able to stimulate smooth muscle cell and LLC growth in vitro and displayed tumorigenic properties in vivo. Interestingly, these effects were strongly inhibited when DC supernatants were preincubated with an anti-AREG blocking antibody. The combined action of ATP and LPS would be able to generate semimature tolerogenic DCs that additionally secrete angiogenic and tumorigenic factors.
Recently, a study has described a new mechanism of regulation of DC differentiation and DC properties by adenosine.38 Furthermore, Novitsky et al have shown that adenosine-differentiated DCs acquire a specific phenotype associated with proangiogenic and proinflammatory properties, immune suppression, immune tolerance, and polarization of the immune response to Th2.38 It has recently been shown that adenosine induces the semimaturation of murine DCs via the A2B receptor.39 Our pharmacologic data suggested the involvement of P2Y11 receptor in human DCs and the involvement of P2Y2 and A2B receptors in mouse DCs. Indeed the effect of UTP on AREG secretion by mouse DCs supported an involvement of P2Y2 receptor, whereas the agonist effect of NECA and the antagonist effect of MRS1754 in BMDCs were compatible with A2B involvement. The effect of ATP and ATPγS on AREG secretion by BMDCs would be partly due to their degradation into adenosine. The absence of P2Y11 ortholog in mouse would be compensated in murine DCs by a direct action of ATP and ATPγS on P2Y2 receptor and an indirect action on A2B receptor through their degradation.
AREG, a member of the EGF family, has been described as a mitogenic factor of vascular smooth muscle cells.13 Interestingly AREG is commonly overexpressed in cancerous tissues such as human colon, stomach, breast, and pancreas, in which the level of AREG is correlated with tumor progression and poor patient survival.40-42 The secretion of AREG by immune cells has been poorly described so far. Johansson and colleagues have shown that AREG secretion by T cells is induced by the adenosine 3′,5′-monophosphate pathway.43 Furthermore, recently it has been described that eosinophils are a source of AREG when they are stimulated with GM-CSF.44 EGFR receptor, which binds AREG, is expressed by various cell types in solid tumor including tumor cells, tumor endothelial cells, and α-SMA–positive cells such as myofibroblasts, myoepithelial cells, and vascular smooth muscle cells.45,46 AREG secreted by immune cells such as DCs might be able to stimulate tumor growth through a direct action on tumor cells and an effect on the tumor microenvironment, in particular the vasculature. Both mechanisms would be involved in the action of ATP, because we have demonstrated that AREG secreted by ATP-treated DCs increases smooth muscle cell and LLC growth in vitro and in vivo the number of α-SMA–positive cells, which are important in vessel maturation. Indeed angiogenesis does not depend only on endothelial cell invasion but also requires vessel stabilization by pericytes and smooth muscle cells. Recent studies have shown that tumor progression requires mature vessels and a cooperation between VEGF acting on endothelial cells and factors such as platelet-derived growth factor acting on pericytes/smooth muscle cells.47 Our study suggests that AREG might play a similar role.
The effect of ATP on tumor progression remains controversial. It was recently shown that repeated intraperitoneal injections of high concentrations of ATP (50mM) inhibited the growth of A375 melanoma cells.48 ATP is also known to modulate inflammation by triggering IL-1 maturation.49 But ATP is also known to induce shedding of matrix metallopeptidase 950 and indoleamine dioxygenase expression,7 which allow tumor progression.
Our study thus defines DCs as a major source of AREG that could contribute to their tumorigenic properties in inflammatory conditions and extracellular ATP, which is known to be present in the tumor microenvironment, as an important regulator of AREG production. Targeting ATP receptors expressed on DCs or anti-AREG blocking antibodies could have a therapeutic potential in cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by an Action de Recherche Concertée of the Communauté Française de Belgique, by the Belgian Program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Federal Service for Science, Technology and Culture, by grants of the Fonds de la Recherche Scientifique Médicale (FRSM), the Fonds d'Encouragement à la Recherche (FER), the Fonds Emile DEFAY, the Fonds de la Recherche Scientifique Médicale of Belgium, the Walloon Region, and the LifeSciHealth program of the European Community (grant LSHB-2003-503337). D.C. is Research Associate of the Fonds National de la Recherche Scientifique (FNRS). N.B. is supported by the Fonds National de la Recherche Scientifique/FRIA, Belgium.
Authorship
Contribution: N.B. designed and performed the research, analyzed the data, and wrote the paper; L.D.P. performed the research; J.-M.B. wrote the paper; and D.C. designed and analyzed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Communi, Institute of Interdisciplinary Research (IRIBHM), ULB, Bldg C (5th fl), Campus Erasme, 808 Route de Lennik, 1070 Brussels, Belgium; e-mail: communid@ulb.ac.be.

![Figure 5. AREG increased the number of α-SMA-positive vessels in LLC tumors. (A) CD31 and α-SMA staining of LLC tumors. LLC tumor sections were obtained from C57BL/6 mice coinjected with LLC cells and supernatants of BMDCs treated with LPS or LPS+ATPγS. Tumor sections were stained with Hoechst (blue) and with anti-CD31 (green) or anti–α-SMA (red). Arrows indicate CD31/α-SMA–double positive vessels (20×/0.4 numeric aperture [NA] objective). (B) Quantification of α-SMA and CD31–double positive vessels. Supernatants of BMDCs treated with LPS or LPS+ATPγS were incubated in the presence or the absence of an anti–mouse AREG blocking antibody. C57BL/6 mice were then coinjected with LLC cells and 100 μL of BMDC supernatants. At least 7 tumors (10 fields/tumor) were analyzed for each condition. The Student t test was performed using Prism 5.0 software (**P < .01). (C) SMA-positive cells express EGFR. Immunofluorescence analysis demonstrated colocalization of α-SMA expression (red) and EGFR (green) within tumor sections. Nuclei are stained with Hoechst (blue). Arrows indicate α-SMA and EGFR–double positive cells (20×/0.4 NA objective).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/17/10.1182_blood-2010-01-265611/4/m_zh89991059350005.jpeg?Expires=1765201126&Signature=XqkTqjj3O9MY6Z6n7EK00aDqCDb6640UAdmciWd1yfnLZ58kKhjt-vfL~h12qGqrGb6y7IYBfedBLzkJLUn7loqWvRb1M7~eEQv7br-chFDxfMzsU~1BD2APtBTSBJVEzRFv-y4j2NGhOQpeV8bC6IKg6G1wtjJdYZFYcaU7kpJ13SPMFnPBGCgSrWnRLb3XYUbSflvl0aQNsMx-e84c4lto-hsHjmDE~hWm86mFiUqK~EmImrntOSViB6XPEXakQRl0Lu2FckfIVd05jblIYdutF4F9Sji2pyGJCEghWKYfhrpoWrCmY2jMy2od5GytiTXUqas1USccMQLRO1as9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)