To the editor:
We read with great interest the paper by Di Stasi and colleagues1 demonstrating that enforced expression of the chemokine receptor CCR4 improves the homing of CD30-specific chimeric antigen receptor (CAR-CD30)–modified effector T cells to thymus- and activation-regulated chemokine/CC chemokine ligand 17 (TARC/CCL17)–producing CD30+ Hodgkin lymphoma (HL) cells, thereby promoting their antitumor effects in vivo. This elegant work indicates a possible way to exploit the peculiar chemokine milieu of HL for therapeutic purposes.
Reed-Sternberg (RS) cells produce large amounts of TARC/CCL17 and macrophage-derived chemokine (MDC)/CCL22, 2 chemokines capable of recruiting CCR4-expressing cell subsets, including type 2 T helper (Th2) cells and regulatory T cells (Tregs). The relevance of both of these chemokines to the pathobiology of HL is reinforced by the presence of elevated serum levels of TARC and MDC in the great majority of HL patients.2 Although TARC and MDC have been regarded as important determinants of T-cell migration within the HL microenvironment, CCR4− Th2 and Tregs cells are overwhelming in HL lesions, and only a minority of CCR4+ T cells can be usually found by immunohistochemistry in HL-involved tissues.1,3,4 Accordingly, only 11% (± 9%) of peripheral blood T cells from HL patients with active disease expressed CCR4 and slightly migrated in the presence of HL cell lines' supernatants.1
The expression of CCL5/regulated on activation normal T-cell expressed and secreted (CCL5/RANTES) and its receptor CCR5 by RS cells has been recently documented.5 CCL5/RANTES is a chemokine capable of attracting CCR3- and CCR5-expressing cells, including Tregs and Th2 cells, eosinophils, and mast cells.5,6 HL cell lines produce a functional CCL5 capable of inducing a remarkable migration of purified CD4+ T cells, eosinophils, and mast cells.5,6 Interestingly, both CCR3 and CCR5 are expressed on T cells of the HL microenvironment but not on T lymphocytes residing in normal lymph nodes.7 CCR3 is evenly distributed among CD4+ and CD8+ cells, whereas CCR5, like CCR4, is mostly expressed by CD4+ cells.7,8 It is, therefore, tempting to speculate that enforced expression of CCR5 might, in turn, maximize homing of CAR-CD30–modified effector T lymphocytes to RS cells.1
Most interestingly, recent reports demonstrated that chemokines play a critical role also in tumor growth and survival.9,10 In this regard, we and others have shown that RS cells express CCR5 both in vivo and in vitro,5,8 and that its ligand CCL5 has a direct effect on RS cells survival and proliferation (Figure 1).5 This finding reflects a peculiar property of CLL5 since, differently from TARC and MDC, this chemokine was shown to directly regulate growth and survival of tumor cells also in other experimental models, including prostate and breast cancer.9,10 Then CCR5 targeting by effector T cells, through the modalities described by Di Stasi et al,1 may maximize the activity of adoptively transferred antitumor T cells through interference with the autocrine and paracrine growth regulatory loops between RS cells and CCL5.5
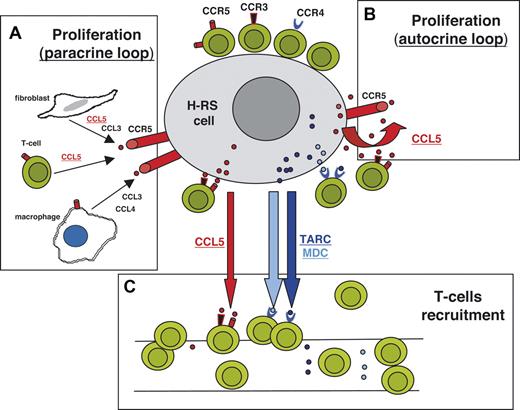
Schematic illustration showing the involvement of TARC, MDC, and CCL5 in microenvironment formation and RS cell growth. (A) The production of CCR5 ligands (CCL5, CCL3, and CCL4) by T cells, macrophages and fibroblasts may contribute to RS cells' proliferation (paracrine loop). (B) CCL5 produced by RS cells may represent an autocrine growth factor. (C) CCL5 produced by RS cells, together with TARC and MDC, may recruit CCR5+, CCR4+, or CCR3+ T cells.
Schematic illustration showing the involvement of TARC, MDC, and CCL5 in microenvironment formation and RS cell growth. (A) The production of CCR5 ligands (CCL5, CCL3, and CCL4) by T cells, macrophages and fibroblasts may contribute to RS cells' proliferation (paracrine loop). (B) CCL5 produced by RS cells may represent an autocrine growth factor. (C) CCL5 produced by RS cells, together with TARC and MDC, may recruit CCR5+, CCR4+, or CCR3+ T cells.
Authorship
Acknowledgments: This work was supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy and the Ministero della Salute, Ricerca Finalizzata FSN, IRCCS, Rome, Italy (D.A. and A.P.) and by a grant from the Ministero della Salute, Rome, within the framework of the Progetto Integrato Oncologia-Advanced Molecular Diagnostics “Multidimensional characterization of tumors” project, and the Ministero della Salute, Ricerca Finalizzata FSN, IRCCS, Rome, Italy (A.C.).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donatella Aldinucci, Experimental Oncology 2, Centro di Riferimento Oncologico, IRCCS, via F. Gallini, 2, 33081 Aviano (PN), Italy; e-mail: daldinucci@cro.it.