In this issue of Blood, Capitani and colleagues report 3 novel and interesting findings that appear highly relevant to the pathophysiology of CLL: (1) that expression of the molecular adaptor p66Shc is substantially reduced or absent in CLL B cells; (2) that p66Shc attenuates BCR signaling, presumably by preventing Syk recruitment and/or activation following BCR engagement; and (3) that reduced expression of p66Shc leads to deregulated expression of antiapoptotic (Bcl-2, Bcl-xL) and proapoptotic (Bax, Bak) Bcl-2 family proteins in CLL B cells.1
Chronic lymphocytic leukemia (CLL) B cells display 2 typical features that are believed to play an important role in the pathogenesis of this disease. The first is overexpression of the antiapoptotic protein Bcl-2, which is considered largely responsible for the extended survival of the leukemic cells. The mechanisms that mediate Bcl-2 overexpression in CLL are still not completely understood, although down-regulation of miR-15 and miR-16, which negatively regulates Bcl-2 at a posttranscriptional level, is a likely explanation in a subset of cases.2 The second feature is frequent expression of nearly identical (stereotyped) B-cell receptors (BCRs) by CLL B cells from different patients, which is generally accepted as evidence that the expansions of the malignant clones are driven, at least initially, by antigen stimulation.3,4
In this issue of Blood, Capitani et al show that expression of Bcl-2 and several other members of this family, as well as signaling through the BCR, are regulated by the adaptor protein Shc.1 This protein exists as 3 isoforms (p46Shc, p52Shc, and p66Shc) that are generated by alternative promoter usage. The p52Shc isoform is mitogenic in T cells and was previously shown by the same authors to couple the activated T-cell receptor to the Ras/MAPK pathway.5 In contrast, p66Shc inhibits this pathway by competitively inhibiting recruitment of p52Shc to the TCR.5
In the current study, Capitani et al show that p66Shc also inhibits activation of ERK, Syk, and Akt in B cells that have been stimulated through the BCR. In turn, inhibition of Syk, Akt, and ERK activation shifts the balance of the BCR signal toward apoptosis rather than increased survival (see figure). Importantly, Capitani et al show that p66Shc expression is significantly reduced in CLL B cells compared with normal B cells, with the lowest levels in cases with unfavorable prognostic features (unmutated Ig VH genes). In contrast, they find that the mitogenic/prosurvival p52Shc isoform is equally expressed in CLL and normal B cells. Although direct evidence from experiments with primary CLL cells is still lacking, these findings suggest that reduced p66Shc expression could enhance antiapoptotic BCR signaling in the malignant B cells and thus favor their growth and survival following antigen exposure in vivo.
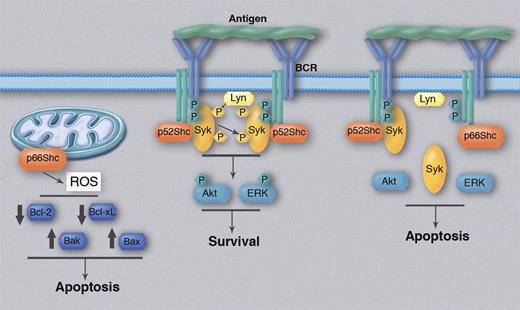
Regulation of BCR signaling and Bcl-2 family protein expression by p66Shc. BCR cross-linking by antigen induces recruitment and activation of Syk, which could be facilitated by the adaptor protein p52Shc. Syk then activates Akt and ERK, which transduce the antiapoptotic BCR signal. p66Shc competitively inhibits recruitment of p52Shc, resulting in less efficient activation of Syk, Akt, and ERK and an alteration in the balance between antiapoptotic and proapoptotic BCR signals in favor of the latter. In parallel, p66Shc can enhance production of reactive oxygen species (ROS) by mitochondria, which could be responsible for the induced changes in the expression of antiapoptotic (Bcl-2, Bcl-xL) and proapoptotic (Bak, Bax) Bcl-2 family members. Phosphorylated proteins are depicted with a “P.” Professional illustration by Marie Dauenheimer.
Regulation of BCR signaling and Bcl-2 family protein expression by p66Shc. BCR cross-linking by antigen induces recruitment and activation of Syk, which could be facilitated by the adaptor protein p52Shc. Syk then activates Akt and ERK, which transduce the antiapoptotic BCR signal. p66Shc competitively inhibits recruitment of p52Shc, resulting in less efficient activation of Syk, Akt, and ERK and an alteration in the balance between antiapoptotic and proapoptotic BCR signals in favor of the latter. In parallel, p66Shc can enhance production of reactive oxygen species (ROS) by mitochondria, which could be responsible for the induced changes in the expression of antiapoptotic (Bcl-2, Bcl-xL) and proapoptotic (Bak, Bax) Bcl-2 family members. Phosphorylated proteins are depicted with a “P.” Professional illustration by Marie Dauenheimer.
Probably an even more important finding of this study is the evidence that p66Shc may be directly responsible for the imbalance in the expression of Bcl-2 family members in CLL B cells. This was elegantly demonstrated by reintroducing p66Shc in primary CLL cells, which resulted in a significant reduction in the expression of antiapoptotic Bcl-2 and Bcl-xL, a concomitant increase in the expression of proapoptotic Bax and Bak, and an increase in the percentage of apoptotic cells. Corresponding changes were observed in B cells from wild-type and p66Shc knockout mice, further suggesting that the imbalance in Bcl-2 family protein expression in CLL B cells may be caused by p66Shc deficiency.
What remains unclear from this study is the mechanism through which p66Shc regulates the expression of Bcl-2 family proteins. One possibility is that p66Shc deficiency could lead to increased tonic BCR signaling, a phenomenon that was recently described in CLL and several other B-cell malignancies and was shown to contribute to the increased apoptosis resistance of the leukemic cells. In favor of this possibility is the observation of the authors that both ligand-dependent and ligand-independent phosphorylation of Syk is enhanced in the absence of p66Shc. However, recently published data suggest that in CLL cells, the antiapoptotic effect of constitutively active Syk is primarily related to changes in the expression of Mcl-1 and Bim, whereas expression of Bcl-2, Bcl-xL, and Bax does not appear to be affected.6 Alternatively, the mechanism through which p66Shc modulates Bcl-2 family protein expression could be related to some other function of this protein. Apart from its role as an adaptor, p66Shc is known to function also as a redox enzyme that enhances reactive oxygen species production by mitochondria.7 Production of reactive oxygen species results in mitochondrial dysfunction, cytochrome-c release, and activation of the caspase cascade, but has also been shown to affect Bcl-2 expression.8
A second unresolved question is the mechanism responsible for the reduced expression of p66Shc in CLL cells. The observation that p66Shc is expressed at higher levels in mutated CLL than in unmutated CLL cells can be taken to suggest that expression of this protein could be modulated by external stimuli from the microenvironment. Identification of stimuli that up-regulate p66Shc may possibly provide a new venue for CLL treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■