Abstract
Fluid shear stress generated by steady laminar blood flow protects vessels from atherosclerosis. Krüppel-like factor 2 (KLF2) and endothelial nitric oxide synthase (eNOS) are fluid shear stress–responsive genes and key mediators in flow anti-inflammatory and antiatherosclerotic actions. However, the molecular mechanisms underlying flow induction of KLF2 and eNOS remain largely unknown. Here, we show a novel role of histone deacetylase 5 (HDAC5) in flow-mediated KLF2 and eNOS expression. We found for the first time that fluid shear stress stimulated HDAC5 phosphorylation and nuclear export in endothelial cells through a calcium/calmodulin-dependent pathway. Consequently, flow induced the dissociation of HDAC5 and myocyte enhancer factor-2 (MEF2) and enhanced MEF2 transcriptional activity, which leads to expression of KLF2 and eNOS. Adenoviral overexpression of a HDAC5 phosphorylation–defective mutant (Ser259/Ser498 were replaced by Ala259/Ala498, HDAC5-S/A), which shows resistance to flow-induced nuclear export, suppressed flow-mediated MEF2 transcriptional activity and expression of KLF2 and eNOS. Importantly, HDAC5-S/A attenuated the flow-inhibitory effect on monocyte adhesion to endothelial cells. Taken together, our results reveal that phosphorylation-dependent derepression of HDAC5 mediates flow-induced KLF2 and eNOS expression as well as flow anti-inflammation, and suggest that HDAC5 could be a potential therapeutic target for the prevention of atherosclerosis.
Introduction
The vascular endothelial cell (EC) lining between the blood and vessel wall is critical to protect against vascular pathogenesis, such as atherosclerosis.1 Fluid shear stress, the frictional dragging force generated by steady laminar blood flow over the surface of the vascular endothelium, plays an important role in enhancing endothelial cell survival, regulating vascular tone, and anti-inflammation and antiatherosclerosis.1-5 These effects occur through flow-dependent induction of certain genes, such as Krüppel-like factor 2 (KLF2).6-9 Krüppel-like factors (KLFs) are a subclass of the zinc finger family of DNA-binding transcription factors, and are expressed in ECs.9-11 Fluid shear stress induces KLF2 expression, which in turn regulates many flow-responsive genes, including endothelial nitric oxide synthase (eNOS).6,7,12,13 Furthermore, KLF2 and eNOS were found to regulate leukocyte adhesion to the endothelium by down-regulating expression of adhesion molecules that recruit leukocytes.6,14-16 Therefore, KLF2 and eNOS serve as fluid shear stress–induced genes that are important in the integration of multiple endothelial functions.9
Recent studies have demonstrated that myocyte enhancer factor-2 (MEF2), a member of the MADS box (MCM1, agamous, deficiens, serum response factor) family of transcription factors that bind to A/T-rich sequences,17 can bind to the KLF2 promoter region and stimulate KLF2 expression.18 MEF2 is also a well-characterized downstream target of fluid shear stress–activated extracellular signal–related kinase 5 (ERK5), a member of the mitogen-activated protein kinase family.19 In contrast, histone deacetylase 5 (HDAC5), one of the class IIa histone deacetylases, serves as a negative regulator of MEF2 transcriptional activity in cardiomyocytes and skeletal muscle cells, regulating muscle differentiation and cardiac growth.20,21 However, whether HDAC5 is involved in flow regulation of MEF2 transcriptional activity and, consequently, expression of KLF2 and eNOS remains unclear.
In this report, we show for the first time that fluid shear stress stimulates the phosphorylation (Ser259/Ser498) and nuclear export of HDAC5. As a consequence, MEF2 transcriptional activity is enhanced and culminates in the induction of KLF2 and eNOS expression and flow.
Methods
Cell culture and flow experiments
Human umbilical vein ECs (HUVECs) were isolated from fresh human umbilical veins and grown in medium 200 with 5% fetal bovine serum and low-serum growth supplement (LSGS; Cascade Biologics Inc).22 Confluent cells cultured in 60-mm dishes were serum-starved for 24 hours and exposed to fluid shear stress as indicated. For the inhibitor studies, cells were pretreated with various inhibitors for 30 minutes in serum-depleted medium. Cells were exposed to laminar flow (shear stress of 24 dyne/cm2) in a cone and plate viscometer.23
Reagents
Protein kinase inhibitors Gö6976, Y-27632, W-12, W-13, KN-62, KN92, KN93, 1,2-bis-(o-Aminophenoxy)ethane-N,N,N',N'-tetraacetic Acid Tetra-(acetoxymethyl) Ester (BAPTA/AM), PP2, PD 98059 compound C, and calmodulin inhibitory peptide were from Calbiochem. Anti–phospho-HDAC5 and anti-HDAC5 were from Signalway Antibody Co Ltd. Anti-eNOS, anti-KLF2 and anti–β-actin antibodies were from Santa Cruz Biotechnology.
Adenovirus constructs and infection
Adenovirus expressing GFP-HDAC5-WT and GFPHDAC5-S/A was generated from pCMV-FLAG-HDAC-WT and pCMV-FLAG-HDAC5-S/A, respectively, using ViraPower Adenoviral Expression System (Invitrogen).24 The infection of ECs with recombinant adenovirus was performed as described previously.25 Briefly, ECs cultured in 60-mm dishes were infected with recombinant adenoviruses at 100 of the multiplicity of infection for 24 hours in growth medium, and then treated with or without inhibitors followed by the application of fluid shear stress.
Transfection and luciferase reporter assay
Transfections for reporter assay were performed with Electroporation (Bio-Rad).22 All transfections were performed in triplicate and represent the mean of at least 3 independent experiments. Cells were stimulated for 8 hours before harvesting, and reporter assays were performed according to the recommendations of the manufacturer of the dual luciferase reporter assay (Promega).
Immunoprecipitation and Western blot analysis
Cells were harvested in lysis buffer and clarified by centrifugation. The protein concentrations in the lysates were determined using the Bradford method (Bio-Rad). Anti-mouse flag agarose beads (Sigma-Aldrich) were used to pull down the protein. Afterward, beads were washed with lysis buffer, and immune complexes were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Total cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes, and the membranes were incubated with appropriate primary antibodies. After incubating with fluorescence-conjugated secondary antibodies, immunoreactive proteins were visualized by Odyssey Infrared Imaging System (LI-COR Biotechnology). Densitometric analyses of immunoblots were performed with Odyssey software (LI-COR Biotechnology). Results were normalized by arbitrarily setting the densitometry of control sample to 1.0.
HDAC5 subcellular localization immunofluorescence study
For analysis of subcellular localization of HDAC5 in ECs, HUVECs were infected with adenovirus encoding GFP-HDAC5-WT or GFP-HDAC5-S/A on gelatin-coated dishes containing medium 200 supplemented with 5% fetal bovine serum and LSGS. After overnight culture, the cells were cultured in serum-free medium for 18 hours. The cells were stimulated with fluid shear stress for the indicated time or exposed to various inhibitors for 30 minutes before stimulation by fluid shear stress. The cells were washed with PBS and fixed with 3.7% formaldehyde in PBS at room temperature. Images were captured using a fluorescence microscope (magnification: ×60, numeric aperture: 0.90, Olympus BX51; Olympus) using an RT Color camera (Model 2.2.1; Diagnostic Instruments Inc) and SPOT Imaging software advanced (Diagnostic Instruments Inc).
RNA isolation, reverse-transcription PCR, and quantitative real-time reverse-transcription PCR
Total RNA was isolated from cultured ECs using a Total RNA Isolation Kit (QIAGEN).22 First-strand cDNA was synthesized with the SuperScript Preamplification System (Gibco-BRL). cDNA was amplified by polymerase chain reaction (PCR) for 30 cycles (Applied Biosystems). Primer sequences for human KLF2 are 5′-AGACCTACACCAAGAGTTCGCATC-3′ and 5′-ATCGCACAGATGGCACTGGAATG-3′; and for human eNOS are 5′-GCTGCGCCAGGCTCTCACCTTC-3′ and 5′-GGCTGCAGCCCTTTGCTCTCAA-3′. Human glyceraldehyde-3-phosphate dehydrogenase served as an internal control. Real-time PCR was performed using SYBR Green (Bio-Rad) and the Bio-Rad MyiQ2 2-Color Real-Time PCR Detection System. Primer sequences for human KLF2 are 5′-GCACCGCCACTCACACCTG-3′ and 5′-CCGCAGCCGTCCCAGTTG-3′; and for human eNOS are 5′-ATGTTTGTCTGCGGCGATGTTAC-3′ and 5′-ATGCGGCTTGTCACCTCCTG-3′. Human glyceraldehyde-3-phosphate dehydrogenase served as an internal control. Experiments were repeated 3 times, in duplicate.
Monocyte adhesion
HUVECs were infected with Ad-LacZ (control) or Ad-HDAC5-S/A. Then cells were exposed to either flow or static condition for 24 hours followed by treatment with 10 ng/mL tumor necrosis factor-α (TNFα) for 6 hours. Then the media were removed, and U937 cells were added to dishes and incubated for 10 minutes at 37°C. Then unbound cells in the dishes were removed by washing 3 times with serum-free medium. The adherent cells were counted in 5 randomly selected optical fields in each well. Phase-contrast microphotographs of the cells in plates were taken with Zeiss Axiovert 40C microscope (magnification: ×10; numeric aperture: 0.25; Carl Zeiss MicroImaging Inc) using a Canon A640 camera (Canon USA Inc).
Data analysis
All data represent the mean plus or minus standard error of the mean of at least 3 separate experiments. Differences between groups were tested for statistical significance using Student t test or analysis of variance. Statistical significance was set at P less than .05.
Results
Fluid shear stress stimulates HDAC5 phosphorylation in ECs
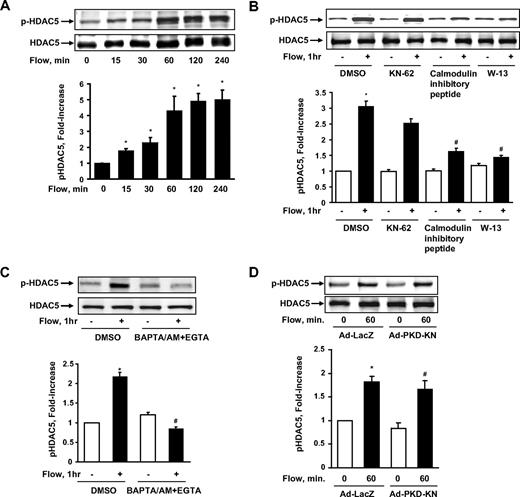
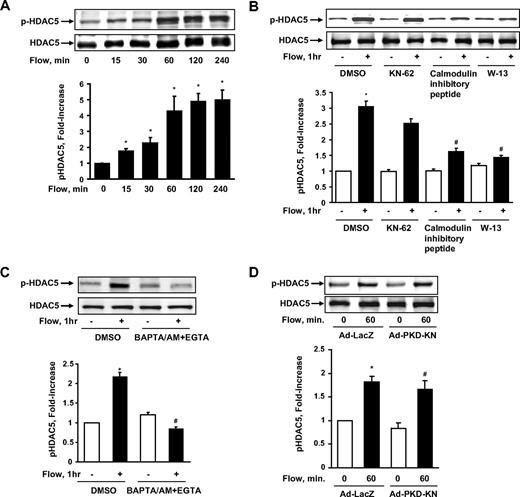
To determine whether HDAC5 is involved in flow signaling in ECs, we exposed human umbilical vein ECs (HUVECs) to fluid shear stress for different time points.23 Hereafter the term “flow” denotes “steady laminar flow” (fluid shear stress = 12 dyne/cm2), which is within the physiologic range of 10 to 40 dyne/cm2, (1 dyne = 1 × 10−5 N) except where indicated specifically. The cell lysates were collected, and HDAC5 phosphorylation on Ser259 and Ser498 (p-HDAC5) was analyzed by immunoblotting with phospho-specific HDAC5 antibody.22 As shown in Figure 1A, flow stimulated HDAC5 phosphorylation in a time-dependent manner and reached the peak level at approximately 1 hour. The total level of HDAC5 was unchanged during flow stimulation. We also detected a similar time-dependent phosphorylation of HDAC5 by flow in bovine aortic ECs (data not shown). To assess the physiologic relevance of this finding, we also examined the phosphorylation of HDAC5 in response to different pattern of flow, which include disturbed flow and changing from low laminar shear stress (2 dyne/cm2) to high laminar shear stress (24 dyne/cm2; supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article). The results showed that HDAC5 cannot be phosphorylated under disturbed flow or low shear stress (supplemental Figure 1), but can still be phosphorylated by high laminar shear stress even after 24-hour adaptation of low laminar shear stress (supplemental Figure 2).
Fluid shear stress stimulated HDAC5 phosphorylation through a Ca2+/calmodulin–dependent pathway in endothelial cells. (A) HUVECs were exposed to flow for 0, 15, 30, 60, 120, and 240 minutes. (B-C) HUVECs were exposed to either flow or static condition for 1 hour with the pretreatment of dimethyl sulfoxide (DMSO), KN-62 (30μM), CaM inhibitory peptide (60nM), W-13 (100μM), and BAPTA/AM (30μM) + EGTA (2μM) for 30 minutes. (D) HUVECs were infected with Ad-PKD-KN or Ad-LacZ for 24 hours before the 1-hour flow or static treatment. HDAC5 phosphorylation (Ser259 and Ser498) and HDAC5 were probed by phospho-specific HDAC5 antibody and HDAC5 antibody. *P < .05 compared with control. #P < .05 compared with cells treated with flow + DMSO/Ad-LacZ. Error bars represent ±SEM.
Fluid shear stress stimulated HDAC5 phosphorylation through a Ca2+/calmodulin–dependent pathway in endothelial cells. (A) HUVECs were exposed to flow for 0, 15, 30, 60, 120, and 240 minutes. (B-C) HUVECs were exposed to either flow or static condition for 1 hour with the pretreatment of dimethyl sulfoxide (DMSO), KN-62 (30μM), CaM inhibitory peptide (60nM), W-13 (100μM), and BAPTA/AM (30μM) + EGTA (2μM) for 30 minutes. (D) HUVECs were infected with Ad-PKD-KN or Ad-LacZ for 24 hours before the 1-hour flow or static treatment. HDAC5 phosphorylation (Ser259 and Ser498) and HDAC5 were probed by phospho-specific HDAC5 antibody and HDAC5 antibody. *P < .05 compared with control. #P < .05 compared with cells treated with flow + DMSO/Ad-LacZ. Error bars represent ±SEM.
Ca2+/calmodulin-dependent pathway mediates fluid shear stress–induced HDAC5 phosphorylation
To determine the molecular mechanisms by which flow stimulates HDAC5 phosphorylation, we treated HUVECs with various protein kinase inhibitors to see whether they could block flow-induced HDAC5 phosphorylation. The inhibitors and their inhibitory effects are shown in the supplemental Table 1. BAPTA/AM + EGTA (ethyleneglycoltetraacetic acid), W-13, and CaM inhibitory peptide blocked HDAC5 phosphorylation induced by flow, whereas PP2, Gö6976, Y-27632, compound C, and PD 98059 had no inhibitory effect on flow-induced HDAC5 phosphorylation. Figure 1B-C shows representative blots of flow-induced HDAC5 phosphorylation inhibited by CaM inhibitory peptide, W-13, and BAPTA/AM + EGTA but not Ca2+/calmodulin–dependent kinase (CaMK) II inhibitor KN62. Our previous studies showed that protein kinase D (PKD) is able to phosphorylate HDAC5 in response to vascular endothelial growth factor in ECs.22 We then examined the role of PKD in flow-induced HDAC5 phosphorylation using the dominant negative form of PKD-encoded adenovirus (Ad-PKD-KN). As shown in Figure 1D, Ad-PKD-KN has no inhibitory effect on flow-induced HDAC5 phosphorylation. Taken together, these results suggest that flow-induced HDAC5 phosphorylation is Ca2+/calmodulin–dependent.
Fluid shear stress induces HDAC5 nuclear export
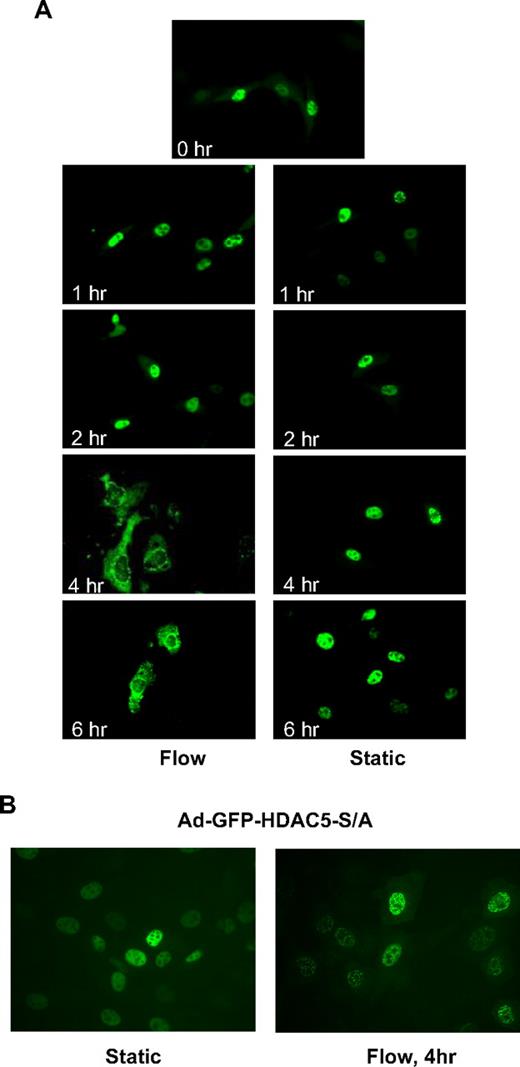
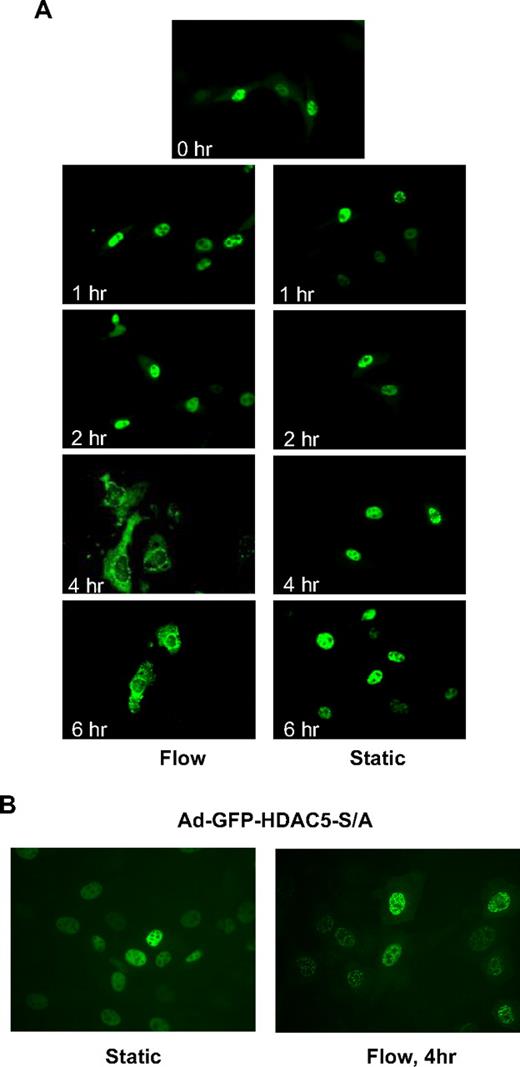
It has been shown that phosphorylation of class IIa HDACs creates binding sites for the 14-3-3 chaperone protein that drive HDACs export from the nucleus to the cytoplasm.20,21 To determine whether flow induces HDAC5 nuclear export, we infected HUVECs with adenoviruses expressing GFP-HDAC5 wild-type (Ad-GFP-HDAC5-WT) to visualize the localization of HDAC5 with fluorescence microscopy as described previously.22 As shown in Figure 2A, HDAC5 localized in the nuclei under static conditions as well as after 1- and 2-hour flow. However, HDAC5 started to appear in the cytoplasm after 4- or 6-hour flow. The time lag of nuclear export likely reflects the requisite time required for binding and export of targets by chaperone proteins (eg, 14-3-3), and nuclear export protein exportin 1.20 Consistent with the phosphorylation data, disturbed flow did not drive HDAC5 out the nuclei after 4 hours (supplemental Figure 3). These results indicate that fluid shear stress induces HDAC5 nuclear export in ECs in a time-dependent manner.
Fluid shear stress induced phosphorylation-dependent HDAC5 nuclear export. (A-B) HUVECs were infected with Ad-GFP-HDAC5 or Ad-GFP-HDAC5-S/A. Twenty-four hours later, cells were conditioned with flow (left lane) or static (right lane) for the time indicated in each image. Images were captured at a magnification of ×60, using a fluorescence microscope.
Fluid shear stress induced phosphorylation-dependent HDAC5 nuclear export. (A-B) HUVECs were infected with Ad-GFP-HDAC5 or Ad-GFP-HDAC5-S/A. Twenty-four hours later, cells were conditioned with flow (left lane) or static (right lane) for the time indicated in each image. Images were captured at a magnification of ×60, using a fluorescence microscope.
HDAC5 phosphorylation on Ser259/498 sites is required for its nuclear export in response to flow
To determine whether flow-dependent phosphorylation of HDAC5 at Ser259/498 residues is required for HDAC5 nuclear export in ECs, we studied the subcellular localization of GFP-HDAC5-S/A, a mutant construct in which both serines 259 and 498 are mutated to alanine. HUVECs were infected with Ad-GFP-HDAC5-WT or Ad-GFP-HDAC5-S/A. Figure 2B shows that both GFP-HDAC5-WT and GFP-HDAC5-S/A were localized in the nuclei under static conditions in ECs. After 4 hours of flow stimulation, GFP-HDAC5-WT was translocated into the cytoplasm, whereas GFP-HDAC5-S/A remained in the nuclei after flow stimulation. These data indicate that flow-dependent phosphorylation of HDAC5 at Ser259/498 is critical for its nuclear export in ECs.
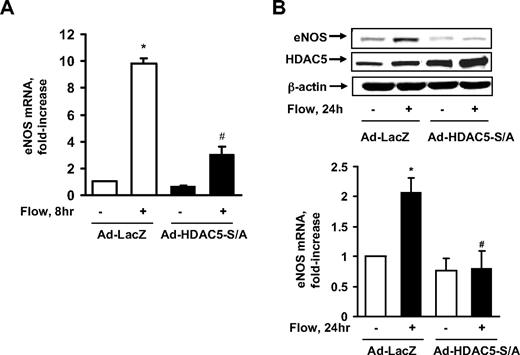
HDAC5 regulates flow-induced KLF2 expression in ECs
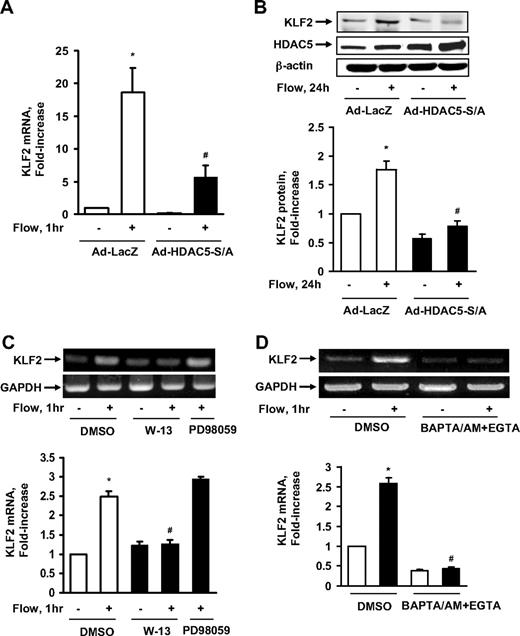
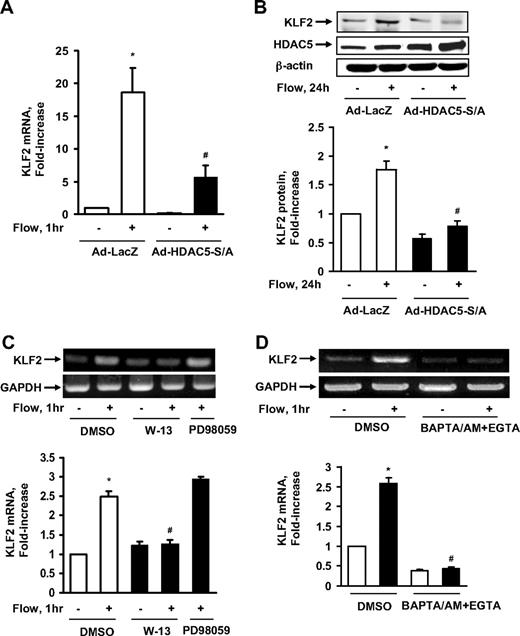
Class IIa HDACs play an important role as transcriptional repressors for gene expression.20 To determine the role of HDAC5 in flow-induced atheroprotective gene expression, we adenovirally expressed the HDAC5-S259A/S498A mutant to see whether it affected the mRNA level of KLF2, a MEF2-dependent and flow-responsive gene.9,18 We subjected ECs to flow for 1 hour, as this time point was found to maximally induce KLF2 mRNA expression (supplemental Figure 4) and HDAC5 phosphorylation. The real-time PCR results in Figure 3A show that flow enhanced KLF2 mRNA expression, whereas HDAC5-S/A dramatically decreased KLF2 mRNA expression induced by flow. The level of KLF2 protein expression induced by flow was also inhibited by HDAC5-S/A as shown in Figure 3B. Consistent with the role of Ca2+/calmodulin in flow-induced HDAC5 phosphorylation, W-13 and BAPTA/AM + EGTA also inhibited the induction of KLF2 mRNA by flow (Figure 3C-D). These results suggest that although HDAC5 can repress KLF2 expression under static conditions, flow induces KLF2 expression by removing the inhibitory effect of HDAC5 on KLF2 expression.
HDAC5 down-regulated flow-mediated KLF2 expression. (A-B) HUVECs were exposed to 1-hour flow or to static conditions after 24-hour infection with Ad-HDAC5-S/A or Ad-LacZ (control). KLF2 mRNA levels were assayed by real-time reverse-transcription (RT)–PCR (A) and protein levels were detected by KLF2 antibody (B). (C-D) HUVECs were exposed to flow or to static conditions after pretreatment with DMSO (control), W-13 (100μM), or PD 98059 (another control; 30μM) for 30 minutes. KLF2 mRNA level were assayed by RT-PCR. Vertical lines have been inserted to indicate repositioned gel lanes. *P < .05 compared with control. #P < .05 compared with that treated with flow + Ad-LacZ or DMSO. Error bars represent ±SEM
HDAC5 down-regulated flow-mediated KLF2 expression. (A-B) HUVECs were exposed to 1-hour flow or to static conditions after 24-hour infection with Ad-HDAC5-S/A or Ad-LacZ (control). KLF2 mRNA levels were assayed by real-time reverse-transcription (RT)–PCR (A) and protein levels were detected by KLF2 antibody (B). (C-D) HUVECs were exposed to flow or to static conditions after pretreatment with DMSO (control), W-13 (100μM), or PD 98059 (another control; 30μM) for 30 minutes. KLF2 mRNA level were assayed by RT-PCR. Vertical lines have been inserted to indicate repositioned gel lanes. *P < .05 compared with control. #P < .05 compared with that treated with flow + Ad-LacZ or DMSO. Error bars represent ±SEM
Flow induces the dissociation of HDAC5 and MEF2C in ECs
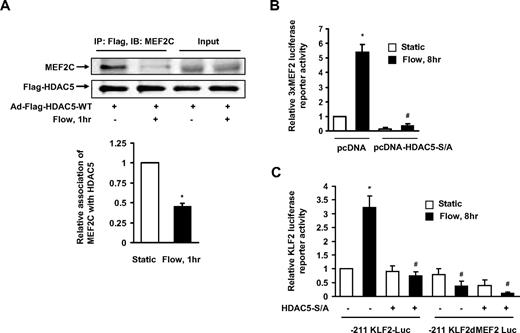
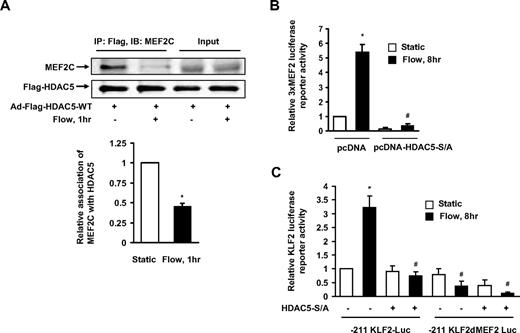
MEF2C, which is a main isoform of MEF2 in ECs, serves as a transcriptional regulator of KLF2 expression under fluid shear stress.7,18 To better understand the detailed mechanism of how HDAC5 regulates KLF2 expression, we examined the interaction between HDAC5 and MEF2C in ECs with or without flow stimulation. HUVECs were infected with adenoviruses expressing wild-type Flag-HDAC5 24 hours before onset of 2-hour flow. As shown in Figure 4A, MEF2C coimmunoprecipitated with HDAC5 under static condition. The association of HDAC5 and MEF2C was reduced after the onset of flow stimulation. This result indicates that although HDAC5 binds to MEF2C in ECs under static condition, flow induces the dissociation of HDAC5 and MEF2C, which may facilitate MEF2C function.
HDAC5 negatively regulated MEF2 transcriptional activation and KFL2 expression. (A) HUVECs were infected with Ad-Flag-HDAC5. Twenty-four hours later, cells were conditioned under static conditions (first lane) or flow (second lane) for 1 hour. Immunoprecipitation was performed with Flag antibody–conjugated agarose. MEF2C and Flag-HDAC5 were detected by probing with anti-MEF2C and anti-Flag antibodies. (B-C) HUVECs were transfected with 3xMEF2-luciferase or −221/+1 KLF2 promoter or −221/+1 MEF2 binding site–deleted KLF2 promoter reporter gene together with HDAC5-S/A or pcDNA as control. Twenty-four hours later, cells were conditioned with static or flow for 8 hours. Luciferase activities were assayed by Promega Luciferase assay kit. *P < .05 compared with control (static). #P < .05 compared with that treated with flow + pcDNA. Error bars represent ±SEM.
HDAC5 negatively regulated MEF2 transcriptional activation and KFL2 expression. (A) HUVECs were infected with Ad-Flag-HDAC5. Twenty-four hours later, cells were conditioned under static conditions (first lane) or flow (second lane) for 1 hour. Immunoprecipitation was performed with Flag antibody–conjugated agarose. MEF2C and Flag-HDAC5 were detected by probing with anti-MEF2C and anti-Flag antibodies. (B-C) HUVECs were transfected with 3xMEF2-luciferase or −221/+1 KLF2 promoter or −221/+1 MEF2 binding site–deleted KLF2 promoter reporter gene together with HDAC5-S/A or pcDNA as control. Twenty-four hours later, cells were conditioned with static or flow for 8 hours. Luciferase activities were assayed by Promega Luciferase assay kit. *P < .05 compared with control (static). #P < .05 compared with that treated with flow + pcDNA. Error bars represent ±SEM.
HDAC5 regulates flow-induced MEF2C transcriptional activation and KLF2-promoter activity
To determine the functional role of the association and dissociation between HDAC5 and MEF2C before and after flow stimulation, we assessed the effect of HDAC5 on MEF2C transcriptional activity. HUVECs were transfected with 3xMEF2-luciferase reporter gene,22 in which 3 tandem repeats of MEF2 sites were located upstream of the thymidine kinase gene promoter, together with HDAC5-WT or HDAC5-S/A. Twenty-four hours later, cells were exposed to flow or static conditions for an additional 24 hours followed by assessment of promoter luciferase activity. As shown in Figure 4B, flow enhanced 3xMEF2-luciferase activity, whereas HDAC5-S/A inhibited the flow-induced MEF2 activation. Similar results were observed in ECs transfected with wild-type or MEF2 site-mutated −221/+1 KLF2 promoter (−221/+1) luciferase reporters18 and HDAC5-WT or HDAC5-S/A (Figure 4C). Collectively, these data indicate that flow-mediated phosphorylation and nuclear export of HDAC5 derepress MEF2C and augment transcriptional activity on target promoters such as KLF2.
HDAC5 regulates flow-induced eNOS expression
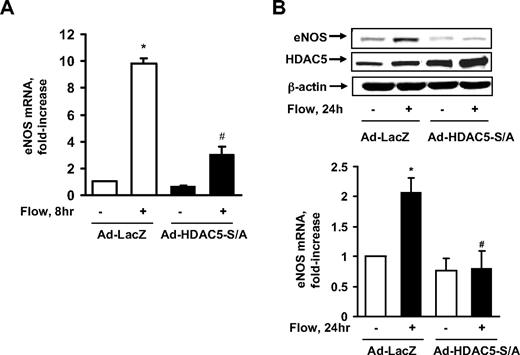
eNOS, which plays a key role in atheroprotection,1 is a KLF2-regulated gene in response to fluid shear stress.6 To determine whether HDAC5 repression of KLF2 has any effect on eNOS expression, we used the adenoviral HDAC5-S259A/S498A mutant to see whether it down-regulates eNOS expression. As the real-time PCR data show in Figure 5A, flow enhanced eNOS mRNA expression, whereas HDAC5-S/A dramatically decreased this eNOS mRNA expression. The level of eNOS protein expression induced by flow was also inhibited by HDAC5-S/A as shown in Figure 5B. Consistent with the protein expression data, nitric oxide production induced by flow was also decreased by HDAC5-S/A (supplemental Figure 5). These results suggest that although HDAC5 decreases expression of the KLF2 downstream gene, eNOS, under static conditions, flow induces KLF2 and eNOS expression by removing the inhibitory effect of HDAC5.
HDAC5 down-regulated flow-mediated eNOS expression. (A-B) HUVECs were exposed to 8 or 24 hours of flow or to static conditions 24 hours after infection with Ad-HDAC5-S/A or Ad-LacZ (control). eNOS mRNA levels were assayed by real-time RT-PCR (A) and protein levels were detected by eNOS antibody (B). Vertical lines have been inserted to indicate repositioned gel lanes. *P < .05 compared with control (static). #P < .05 compared with that treated with flow + Ad-LacZ. Error bars represent ±SEM.
HDAC5 down-regulated flow-mediated eNOS expression. (A-B) HUVECs were exposed to 8 or 24 hours of flow or to static conditions 24 hours after infection with Ad-HDAC5-S/A or Ad-LacZ (control). eNOS mRNA levels were assayed by real-time RT-PCR (A) and protein levels were detected by eNOS antibody (B). Vertical lines have been inserted to indicate repositioned gel lanes. *P < .05 compared with control (static). #P < .05 compared with that treated with flow + Ad-LacZ. Error bars represent ±SEM.
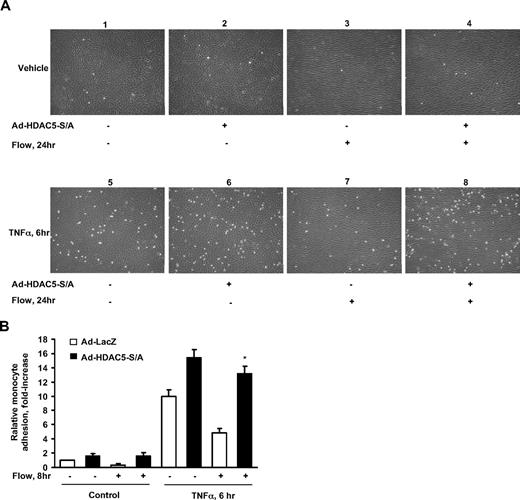
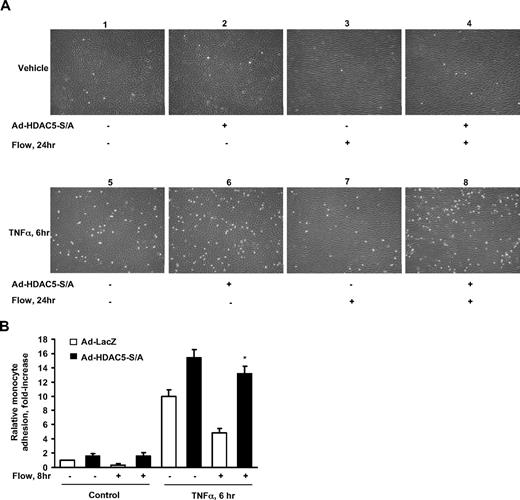
HDAC5 modulates the flow-inhibitory effect on monocyte adhesion to ECs
KLF2 has anti-inflammatory effect in ECs by reducing expression of adhesion molecules that mediate the recruitment of monocytes to ECs.6 To determine the potential role of HDAC5 in regulating the flow-mediated anti-inflammatory effect, we examined monocyte adhesion to ECs challenged with the proinflammatory cytokine tumor necrosis factor alpha (TNFα) under static or flow conditions in the presence or absence of HDAC5-S/A. HUVECs were infected with Ad-HDAC5-S/A or Ad-LacZ for 24 hours before the onset of 24-hour flow followed by TNFα for 6 hours. As shown in Figure 6, flow-stimulated ECs have significantly less monocyte adhesion compared with static cells under TNFα treatment. In contrast, Ad-HDAC5-S/A–infected cells exhibit augmented monocyte adhesion even after 24-hour flow pretreatment. Taken together, our results indicate that flow-mediated effects on HDAC5 critically regulate immune cell adhesion to an activated endothelial monolayer.
HDAC5-dependent pathway modulated flow anti-inflammation in endothelial cells. (A-B) Overexpression of Ad-HDAC5-S/A attenuated flow anti-inflammation. HUVECs were infected with Ad-HDAC5-S/A or Ad-LacZ as control. Twenty-four hours later, cells were subjected to flow or to static conditions for 24 hours followed by the treatment of TNFα or vehicle for 6 hours. U937 monocytes were added to HUVECs and incubated for 30 minutes. HUVECs were washed 3 times. The adherent monocytes were counted in 5 randomly optical fields in each dish. *P < .05, flow + TNFα + Ad-HDAC5-S/A vs flow + TNFα + Ad-LacZ. Error bars represent ±SEM.
HDAC5-dependent pathway modulated flow anti-inflammation in endothelial cells. (A-B) Overexpression of Ad-HDAC5-S/A attenuated flow anti-inflammation. HUVECs were infected with Ad-HDAC5-S/A or Ad-LacZ as control. Twenty-four hours later, cells were subjected to flow or to static conditions for 24 hours followed by the treatment of TNFα or vehicle for 6 hours. U937 monocytes were added to HUVECs and incubated for 30 minutes. HUVECs were washed 3 times. The adherent monocytes were counted in 5 randomly optical fields in each dish. *P < .05, flow + TNFα + Ad-HDAC5-S/A vs flow + TNFα + Ad-LacZ. Error bars represent ±SEM.
Discussion
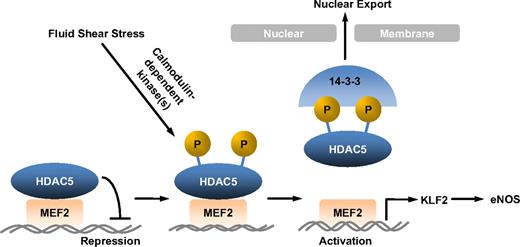
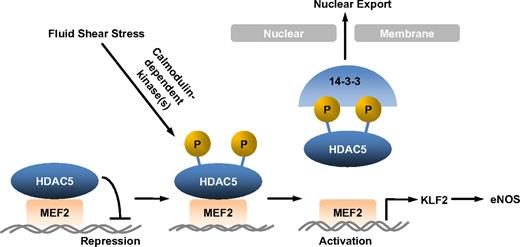
Vascular ECs are constantly exposed to fluid shear stress, the dragging force generated by blood flow. Substantial evidence suggests that fluid shear stress regulates vascular homeostasis and focal distribution of atherosclerosis by influencing endothelial gene expression.2,26 Studying the regulation of flow-mediated genes involved in EC function is therefore important for our understanding of flow atheroprotection. Among many genes induced by flow, KLF2 and eNOS have emerged as key mediators for fluid shear stress atheroprotective effects.9,27,28 However, the molecular mechanisms by which fluid shear stress stimulates KLF2 and eNOS expression in ECs are not fully understood. Our present study has for the first time defined an HDAC5-dependent pathway for regulation of KLF2 and eNOS expression by flow. The majoring findings in this study are (1) fluid shear stress stimulates phosphorylation and nuclear export of HDAC5 in ECs in a Ca2+/CaM-dependent manner, (2) phosphorylation of HDAC5 releases its repression on MEF2 (a critical transcription factor for KLF2 expression) and permits MEF2 activation, (3) flow-induced HDAC5 phosphorylation mediates KLF2 transcription and eNOS expression, and (4) HDAC5-dependent pathway modulates flow anti-inflammation in ECs (Figure 7).
Model for HDAC5 modulated flow-induced KLF2 and eNOS expression. Fluid shear stress stimulates HDAC5 phosphorylation and nuclear export through a Ca2+/calmodulin-dependent pathway, freeing MEF2 to activate KLF2 expression and its downstream gene expression.
Model for HDAC5 modulated flow-induced KLF2 and eNOS expression. Fluid shear stress stimulates HDAC5 phosphorylation and nuclear export through a Ca2+/calmodulin-dependent pathway, freeing MEF2 to activate KLF2 expression and its downstream gene expression.
Histone acetylation/deacetylation is critical for the control of gene expression. Histone acetyltransferases enhance transcription through acetylation of lysine residue on core histones, resulting in relaxation of nucleosomes; conversely, HDACs antagonize this activity and repress transcription.29 HDAC5 is a member of class IIa HDAC family. It has been shown that HDAC5 blocks myogenesis by associating with and inhibiting the activity of MEF2 transcriptional factor.30,31 However, the possible involvement of HDAC5 and fluid shear stress signal transduction and gene regulation is not known. In this study, we demonstrate for the first time that fluid shear stress stimulates HDAC5 phosphorylation and nuclear export in ECs. Several protein kinases including CaMKs and PKD that are responsible for HDAC5 phosphorylation have recently been identified.29 The studies of HDAC5 in skeletal muscle cells reveal that CaMKs regulates HDAC5 phosphorylation and nuclear export.20 Recently, we and others have reported that PKD stimulates HDAC5 phosphorylation in ECs and cardiomyocytes in response to vascular endothelial growth factor22 and alpha-adrenergic receptor agonist phenylephrine,32 respectively. Our present study, however, shows that PKD is not involved in flow-induced HDAC5 phosphorylation, because inhibiting PKD with adenoviral overexpression of PKD dominant-negative mutant did not affect HDAC5 phosphorylation in ECs by flow. Instead, our results show that flow-induced HDAC5 phosphorylation is Ca2+/calmodulin–dependent because chelating intracellular Ca2+ or inhibiting calmodulin blocks HDAC5 phosphorylation by flow. Experiments with the pharmacologic inhibitors also excluded the involvement of CaMK-II in flow-stimulated HDAC5 phosphorylation, suggesting that there might be a specific Ca2+/calmodulin–dependent protein kinase that is responsible for flow-dependent HDAC5 phosphorylation in ECs. Additional studies to define such a protein kinase would help us in further understanding fluid shear stress signal transduction and gene regulation.
KLF2, a zinc finger domain–containing transcription factor, was first identified as a homologous protein of erythroid Krüppel-like factor.10 Increasing evidence suggests that KLF2 plays a critical role in vascular biology. KLF2 regulates leukocyte adhesion to the endothelium, endothelial proliferation, migration and angiogenesis, and expression of factors such as eNOS implicated in controlling vasoreactivity and vascular tone.9,28 Most importantly, KLF2 has recently been defined as a flow-responsive gene.9,28 Dekker et al first showed that fluid shear stress up-regulates expression of KLF2 in ECs.12 It has been demonstrated that MEF2 is a key transcription factor to drive KLF2 expression.19 Recent studies further show that flow stimulates mitogen-activated protein kinase kinase 5/ERK5/MEF2 pathway and mediates KLF2 expression.7 In addition to this direct up-regulation of KLF2 expression through mitogen-activated protein kinase kinase 5/ERK5/MEF2 pathway, our studies showed, for the first time, that HDAC5 serves as a negative regulator of flow-mediated KLF2 expression. We found that flow-stimulated phosphorylation and nuclear export of HDAC5 resulted in the dissociation of HDAC5 with MEF2C, which releases the inhibition on MEF2 and permits MEF2-dependent KLF2 gene transcription. HDAC5-S/A mutant blocked both the 3xMEF2 luciferase and KLF2 promoter luciferase activity even under flow stimulation, indicating that flow-induced HDAC5 phosphorylation on both Ser259 and Ser498 sites is a key step in regulating MEF2 transcriptional activity and KLF2 expression. Previously, Kumar et al reported that, in the context of cytokine stimulation, HDAC4 and HDAC5 cooperated with p65/NF-κB to inhibit MEF2 transcriptional activity and, consequently, KLF2 expression.18 Our results also support the importance of the HDAC-mediated inhibition of MEF2 in regulating KLF2 expression. Specifically, we found that flow induces KLF2 through stimulating HDAC5 phosphorylation and nuclear export to release its suppressive effect on MEF2 transcriptional activation, which provides insight into flow-mediated KLF2 expression. Collectively, our work and that of Kumar et al identify the importance of the HDAC-MEF2 axis as critical in coordinating KLF2 expression in response to distinct physiologically relevant stimuli.
Steady laminar flow has beneficial effects on endothelial cell function including inhibition of platelet aggregation, low-density lipoprotein uptake, adhesion molecule expression, and vascular smooth muscle cell proliferation, as well as enhancing endothelial cell survival.26 Many of these effects are related to increased nitric oxide (NO) production by ECs exposed to flow.2 A decrease in the bioavailability of NO is a characteristic feature in patients with coronary artery disease and aggravates the development of atherosclerotic lesions.33,34 Flow stimulates production of NO via endothelial nitric-oxide synthase (eNOS) both in cultured ECs and in intact vessels.3 We and others have previously shown that fluid shear stress not only acutely stimulated eNOS activation but also chronically induced eNOS gene expression in ECs.23,35,36 However, the exact mechanisms by which steady laminar flow mediates eNOS expression and atheroprotection have remained poorly understood. Studies by SenBanerjee et al identified KLF2 as a potential inducer of eNOS expression.6 A crucial role of KLF2 in flow-mediated eNOS expression is further substantiated by the observations that knockdown of KLF2 reduces expression of eNOS under flow conditions.13,15 In this study, we show that HDAC5 phosphorylation is required for both KLF2 and eNOS up-regulation by flow, which results in the increase of NO production, suggesting an important role for HDAC5 in regulation of flow-mediated atheroprotective genes. Previous studies showed that flow-induced NO production contributes to the inhibition of monocyte adhesion on ECs.14 However, recent studies strongly support the idea that KLF2 down-regulates the monocyte-EC interaction through inhibiting the expression of cytokine-induced adhesion molecules, which suggested that KLF2 plays a major role in the endothelial anti-inflammation.6,15,16 In agreement with these concepts, our studies show that flow-mediated KLF2 expression and anti-inflammatory effect are through an HDAC5 phosphorylation–dependent pathway, in which HDAC5-S/A dramatically increases monocyte adhesion to ECs even under flow conditions. The connection between flow-induced HDAC5 phosphorylation pathway to KLF2 expression as well as to decreased monocyte adhesion suggests a potential mechanism of flow-induced anti-inflammation. In addition, recent studies by Atkins et al show that the heterozygous deficiency of KLF2 increased diet-induced atherosclerosis in ApoE−/− mice, representing the first in vivo evidence that suggested a role for KLF2 in atheroprotection.27 It would be interesting to further investigate the involvement of HDAC5 phosphorylation-dependent pathway through regulation of KLF2 and eNOS expression in flow atheroprotection in vivo. Of note, HDAC inhibitors have been proposed as possible therapeutic strategies for various human diseases, including cancer.37 Therefore, it is possible that HDAC5 may emerge as a potential pharmacologic target for improving endothelial function and treating or preventing atherosclerosis.
In summary, we have identified an HDAC5-dependent pathway, through which fluid shear stress induces KLF2 and eNOS expression and promotes endothelial anti-inflammation. These studies unveil a novel role of HDAC5 in regulating EC function in response to hemodynamic forces, and thus provide the foundation for novel approaches for the treatment of disease states characterized by endothelial dysfunction.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Jane Sottile for critical reading of the paper.
This work was partially supported by the American Heart Association Predoctoral Fellowship 0815766D (W.W.), Thomas R. Lee Award from the American Diabetes Association 1-06-CD-13 and the Grant-In-Aid Award from the American Heart Association 0755916T (Z.G.J.), and National Institutes of Health grants HL-080611 (Z.G.J.), HL-76754, and P01-HL48743 (M.K.J.).
National Institutes of Health
Authorship
Contribution: W.W., C.H.H., B.S.J., and C.W. performed experiments; M.K.J. contributed new reagents/analytic tools and critically edited the paper; W.W., C.H.H., B.S.J., C.W., and Z.G.J. analyzed data; and W.W. and Z.G.J. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zheng Gen Jin, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642; e-mail: zheng-gen_jin@urmc.rochester.edu.