To the editor:
Numerous immune regulatory functions have been reported for the IL-17 family of cytokines, and, most notably, IL-17 is involved in inducing and mediating proinflammatory responses. In this context, the role of IL-17 in cancer initiation, growth, and metastasis is very controversial.1 This year, a brief report in Blood concluded that host IL-17 reduces tumor growth and metastasis.2 The authors' conclusions relied entirely on one C57BL/6 mouse–derived adenocarcinoma cell line, MC38, in which they showed a 5-fold or greater enhancement in subcutaneous growth and metastases in IL-17–deficient mice compared with control wild-type mice. Their conclusions also contrasted sharply with 3 important previous papers that suggested that IL-17 promoted tumor growth.3,–5 In particular, having worked extensively with the MC38 experimental tumor, we were surprised at the significant enhancement of MC38 tumor growth in IL-17–deficient mice compared with their wild-type controls.
We have now undertaken many studies of tumor initiation, growth, and metastasis in the same IL-17–deficient mice used by Kryczek (provided by Dr Yoichiro Iwakura) compared with wild-type controls. In particular, we would now like to share our results from an analysis of 3 different sources of MC38 tumor cells and some variants, including the original MC38 cells requested and sourced from Dr Walter Storkus (designated MC38s, University of Pittsburgh School of Medicine) and sourced in the Kryczek paper.2 This study was approved by the Peter MacCallum Cancer Centre (Peter Mac) Animal Ethics Committee.
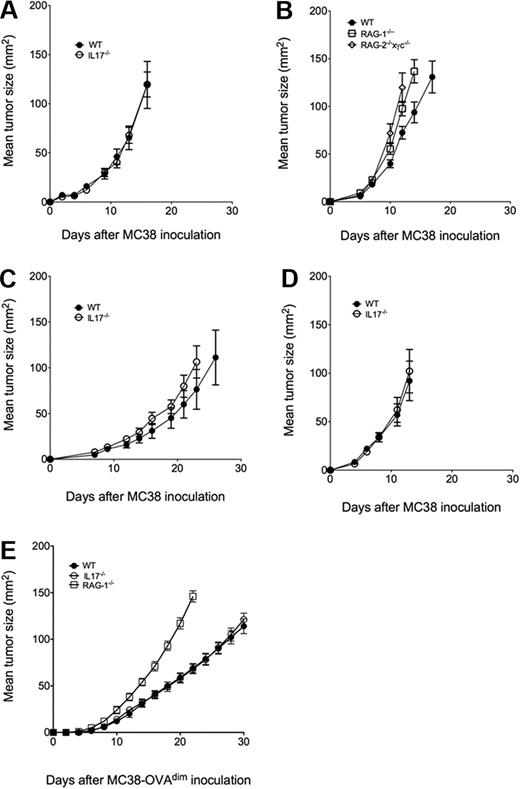
As shown in Figure 1, MC38 cells from the Storkus laboratory (MC38s, Figure 1A-B) and 2 variants independently obtained from within the Peter Mac (MC38a, MC38b, Figure 1C-D) grew equally well subcutaneously in IL-17–deficient mice and wild-type mice. By contrast, MC38s growth was enhanced in either RAG-1- or RAG-2 × γc–deficient mice lacking adaptive immunity or all lymphocytes, respectively (Figure 1B), suggesting that T and NK cells were exerting some host control over MC38s growth, but it was not via their IL-17 production. To further examine MC38 in the context of a stronger adaptive response, we used MC38a-OVAdim cells prepared as previously described.6 We previously showed that CD8+ T cells suppressed MC38a-OVAdim tumors,6 and herein, RAG-1–deficient mice were clearly shown to be far more susceptible to tumor growth than either wild-type or IL-17–deficient mice (Figure 1E).
IL-17 does not regulate MC38 tumor growth and metastasis. Groups of 4 to 8 C57BL/6 wild-type (WT), IL-17–deficient (IL-17−/−), RAG-1–deficient (RAG-1−/−) or RAG-2 × γc–deficient (RAG2−/−×γc−/−) mice were inoculated subcutaneously on the flank with (A-B) 1 × 105 MC38s, (C) 1 × 105 MC38a, (D) 1 × 105 MC38b, or (E) 5 × 105 MC38a-OVAdim tumor cells. Tumor size was measured periodically as the product of 2 tumor diameters using a digital caliper square as indicated. Mean tumor size (in mm2) ± SEM is recorded.
IL-17 does not regulate MC38 tumor growth and metastasis. Groups of 4 to 8 C57BL/6 wild-type (WT), IL-17–deficient (IL-17−/−), RAG-1–deficient (RAG-1−/−) or RAG-2 × γc–deficient (RAG2−/−×γc−/−) mice were inoculated subcutaneously on the flank with (A-B) 1 × 105 MC38s, (C) 1 × 105 MC38a, (D) 1 × 105 MC38b, or (E) 5 × 105 MC38a-OVAdim tumor cells. Tumor size was measured periodically as the product of 2 tumor diameters using a digital caliper square as indicated. Mean tumor size (in mm2) ± SEM is recorded.
In our view it is difficult to reconcile the results obtained by Kryczek et al to what we observe in totally lymphocyte-deficient mice. We have used the same IL-17–deficient mice and 3 independent sources of MC38 cells, including those obtained by both groups from the laboratory of Dr Walter Storkus. Notably, Kryczek measured tumor volume (3 diameters), as opposed to tumor area (mm2) as presented here, but we have additionally made volume measurements (data not shown), and, qualitatively, there is no difference in our conclusions. Perhaps most obvious is the considerable variation in tumorigenic potential of different variants of the MC38 tumor cell line observed even in our experiments between MC38s and MC38a. It is possible that Kryczek and colleagues have developed in culture an unusual variant of MC38s that has a differential growth in IL-17–deficient and wild-type mice. In this case, this may be a valuable variant for the authors and others to study further. Regardless, in our view the readership should consider our contrasting data and continue to ask the question: does IL-17 suppress tumor growth?
Authorship
Acknowledgments: The authors thank Michelle Stirling for maintenance of the mice at the Peter Mac, Dr Yoichiro Iwakura for providing the IL-17A–deficient mice, and Dr Walter Storkus for providing his MC38s cells. This work was supported by the National Health and Medical Research Council of Australia (NH&MRC). M.W.L.T. was supported by a NH&MRC Peter Doherty Fellowship.
Contribution: S.F.N. performed the majority of the experiments; M.W.L.T. directed all of the experiments and co-wrote the paper; and M.J.S. performed experiments and co-wrote the paper. M.J.S. and M.W.L.T. share senior authorship.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: to Mark J. Smyth, Locked Bag 1, A'Beckett St, Cancer Immunology Program, Peter MacCallum Cancer Centre, East Melbourne, VI 8006, Australia; e-mail: mark.smyth@petermac.org; or Michele W. L. Teng, Locked Bag 1, A'Beckett St, Cancer Immunology Program, Peter MacCallum Cancer Centre, East Melbourne, VI 8006, Australia; e-mail: michele.teng@petermac.org.