To the editor:
The groundbreaking innovation of reprogramming adult cells to pluripotency has opened new avenues for patient-tailored treatments. The successful treatment of a humanized sickle cell anemia mouse model with induced pluripotent stem cells (iPSCs) from autologous skin illustrates this principle.1 Human iPSCs can be directed to undergo hematopoietic differentiation in a fashion similar to human embryonic stem cells (hESCs).2,,,–6 However, the globin expression profile of iPSC-derived erythroid cells has not been fully explored and only partial results were mentioned in one study.4 An important question is whether the age of the cells used to derive iPSCs influences developmentally regulated globin expression.
We studied globin expression in erythroid cells derived from 6 different human iPSC lines generated in our laboratory with lentiviral vectors expressing OCT4, NANOG, LIN28, and SOX2 as described.7 Three lines (iPSC-MHF2) were derived from human fetal fibroblasts isolated at 20 weeks' gestation (GM05387, Coriell Institute for Medical Research), and the other 3 (iPSC-OI12) were derived from human mesenchymal stem cells (MSCs) isolated from the spine of a 15-year-old patient.8 Two lines, iPSC-MHF2 C2 and iPSC-OI12 C7, were verified to have normal male karyotype (46X,Y). The ability of each iPSC clone used in this study to give rise to progenies of all 3 germ layers was established by teratoma assays in immunodeficient mice and staining of histologic sections for human microtubule associated protein–2 (MAP-2; ectoderm), human α–smooth muscle actin (SMA; mesoderm), or human α-fetoprotein (AFP; endoderm), as described9 (supplemental Figure 1). The iPSC lines were induced to undergo hematopoietic differentiation as previously described for human ESCs.10
As was previously reported in hESC and iPSC lines,5,10 these 6 iPSC lines exhibited a wide range of hematopoietic differentiation potential, as demonstrated by the percentage expression of hematopoietic markers such as CD45 and glycophorin-A (Figure 1A) and by the formation of hematopoietic colonies (Figure 1B). In 2 of the lines with robust erythroid development (Figure 1C), we studied the globin expression pattern at the mRNA (Figure 1D) and protein levels (Figure 1E). We found that despite the original adult status of the cells from which they were derived, these iPSC-derived erythroid cells expressed mostly embryonic (ϵ) and fetal (γ) globins, similar to ESC-derived erythroid cells,10 consistent with complete reprogramming at the globin locus. This globin phenotype, if maintained in vivo, has an important implication for the treatment of hemoglobinopathies using patient-specific iPSC-derived hematopoietic cells in that correcting the defective β-globin gene may not be necessary. Furthermore, our data highlight the fact that iPSCs are highly heterogeneous in their hematopoietic potential as previously reported by Choi and colleagues.5 While this variation could reflect differential silencing and/or expression of reprogramming factors, it is similar to that observed in human ESC-derived cells, and demonstrates that large numbers of individual iPSC lines need to be screened before drawing conclusions related to the effects of cell origin on differentiation potential. Further studies are needed to dissect these issues in iPSC-derived hematopoietic cells before their anticipated use in humans.
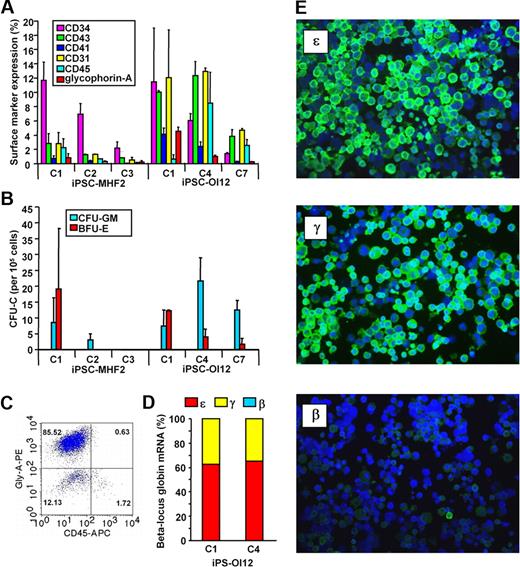
iPSC-derived cells' hematopoietic surface marker expression, clonogenic potential, and globin expression pattern. (A) Hematopoietic surface marker expression by iPSC-derived cells. iPSCs were induced to undergo hematopoietic differentiation by embryoid body (EB) formation as previously described.10 Briefly, undifferentiated iPSC colonies were harvested off the murine embryonic fibroblast feeders and plated onto ultra-low attachment plates (Corning) in the EB medium supplemented with 0.3% methylcellulose (BD Biosciences) for 14 days. At the end of the EB culture, EBs were transferred to Matrigel-coated tissue culture plates (BD Biosciences) and cultured in the hematopoietic growth and expansion medium supplemented with a combination of growth factors. The adherent cells were harvested after 7 days and single-cell suspensions were prepared by enzymatic treatment (0.05% dispase + 0.05% collagenase IA) followed by repeated pipetting. Surface markers were stained using monoclonal antibodies and analyzed using FACSCalibur. Heterogeneity in the response to directed hematopoietic differentiation regimen by individual iPSC lines is shown. Similar results were obtained during spontaneous EB differentiation (data not shown). (B) Clonogenic potential of iPSC-derived cells. Cells prepared as described were cultured either in complete human methycellulose medium (StemCell Technologies) for colony-forming units–granulocyte macrophages (CFU-GM) or in a serum-free semisolid medium10 for burst-forming unit erythroid (BFU-E). The frequency of hematopoietic colonies was enumerated after 14 days of culture. The clonogenic potential differed from line to line. (C) Erythroid differentiation of iPSC. EBs from iPSCs were dissociated by enzymatic treatment followed by forcing through 20G and then 18G needles. Single-cell suspensions were cultured in serum-free media supplemented with erythroid growth factors as previously described.10 Fresh media were added as needed to maintain cell density at ∼ 0.5 × 106/mL through the 14- to 18-day culture period. Robust erythroid development was observed from iPSC-MHF2-C1, iPSC-OI12-C1, or iPSC-OI12-C4. A representative flow cytometric scatter plot of iPSC-OI12-C4–derived cells is shown. Similar to erythroid cells from hESCs,10 iPSC-derived immature and mature erythroid cells do not express CD45. (D) β-Locus globin mRNA expression by iPSC-derived erythroid cells. mRNA was prepared from iPSC-derived erythroid cells and the mRNA levels of all β-like genes were measured by quantitative real-time polymerase chain reaction. High levels of ϵ and γ globin mRNA were detected. (E) Immunostaining of iPSC-derived erythroid cells. Smears prepared from the erythroid cells following serum free liquid culture were stained with monoclonal antibodies against ϵ, γ, or β/δ globin chains, followed by a fluorescein isothiocyanate–conjugated anti-mouse antibody and counterstained with DAPI. Pictures were taken under a Leica DMLB microscope with a 40× PL Fluotar objective using a RT Slider Spot camera. The majority of the erythroid cells expressed ϵ and γ globins, and very few β-producing cells were observed.
iPSC-derived cells' hematopoietic surface marker expression, clonogenic potential, and globin expression pattern. (A) Hematopoietic surface marker expression by iPSC-derived cells. iPSCs were induced to undergo hematopoietic differentiation by embryoid body (EB) formation as previously described.10 Briefly, undifferentiated iPSC colonies were harvested off the murine embryonic fibroblast feeders and plated onto ultra-low attachment plates (Corning) in the EB medium supplemented with 0.3% methylcellulose (BD Biosciences) for 14 days. At the end of the EB culture, EBs were transferred to Matrigel-coated tissue culture plates (BD Biosciences) and cultured in the hematopoietic growth and expansion medium supplemented with a combination of growth factors. The adherent cells were harvested after 7 days and single-cell suspensions were prepared by enzymatic treatment (0.05% dispase + 0.05% collagenase IA) followed by repeated pipetting. Surface markers were stained using monoclonal antibodies and analyzed using FACSCalibur. Heterogeneity in the response to directed hematopoietic differentiation regimen by individual iPSC lines is shown. Similar results were obtained during spontaneous EB differentiation (data not shown). (B) Clonogenic potential of iPSC-derived cells. Cells prepared as described were cultured either in complete human methycellulose medium (StemCell Technologies) for colony-forming units–granulocyte macrophages (CFU-GM) or in a serum-free semisolid medium10 for burst-forming unit erythroid (BFU-E). The frequency of hematopoietic colonies was enumerated after 14 days of culture. The clonogenic potential differed from line to line. (C) Erythroid differentiation of iPSC. EBs from iPSCs were dissociated by enzymatic treatment followed by forcing through 20G and then 18G needles. Single-cell suspensions were cultured in serum-free media supplemented with erythroid growth factors as previously described.10 Fresh media were added as needed to maintain cell density at ∼ 0.5 × 106/mL through the 14- to 18-day culture period. Robust erythroid development was observed from iPSC-MHF2-C1, iPSC-OI12-C1, or iPSC-OI12-C4. A representative flow cytometric scatter plot of iPSC-OI12-C4–derived cells is shown. Similar to erythroid cells from hESCs,10 iPSC-derived immature and mature erythroid cells do not express CD45. (D) β-Locus globin mRNA expression by iPSC-derived erythroid cells. mRNA was prepared from iPSC-derived erythroid cells and the mRNA levels of all β-like genes were measured by quantitative real-time polymerase chain reaction. High levels of ϵ and γ globin mRNA were detected. (E) Immunostaining of iPSC-derived erythroid cells. Smears prepared from the erythroid cells following serum free liquid culture were stained with monoclonal antibodies against ϵ, γ, or β/δ globin chains, followed by a fluorescein isothiocyanate–conjugated anti-mouse antibody and counterstained with DAPI. Pictures were taken under a Leica DMLB microscope with a 40× PL Fluotar objective using a RT Slider Spot camera. The majority of the erythroid cells expressed ϵ and γ globins, and very few β-producing cells were observed.
The online version of this letter contains a data supplement.
Authorship
Contribution: K.-H.C. and A.H. differentiated and evaluated hematopoietic cells; R.K.H. and P.-R.W. generated and characterized iPSCs under the guidance of D.W.R.; and K.-H.C., D.W.R., and T.P. wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thalia Papayannopoulou, MD, DrSci, Professor of Medicine, Division of Hematology, Department of Medicine, University of Washington, 1705 NE Pacific, Rm K243, Box 357710, Seattle, WA 98195-7710; e-mail: Thalp@u.washington.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal