In this issue of Blood, Palumbo and colleagues describe a trial in newly diagnosed patients with MM who are eligible for high-dose therapy. They compare 2 different dosages of double high-dose melphalan (100 mg/m2 vs 200 mg/m2) followed by autologous stem cell transplantation.1 It is concluded that MEL200 is superior to MEL100 with respect to progression-free survival and time to progression, but not overall survival, while treatment-related mortality does not differ.
The concept tested by high-dose melphalan (HDM) is that increasing the dose-intensity of treatment will eradicate resistant cells. The limited nonhematologic toxicity of melphalan has led to its widespread use in myeloablative therapy combined with autologous stem cell support. The regimen can be easily applied, and treatment-related mortality is low. Despite its initial success, HDM with stem cell support has been widely applied only outside the United States, primarily because it requires hospital admission.
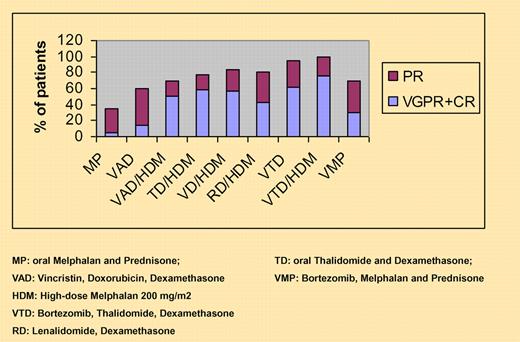
Response rates with current induction regimens before and after high-dose therapy.
Response rates with current induction regimens before and after high-dose therapy.
A key factor to a successful outcome after HDM is achieving complete response before transplantation. Unfortunately, only 15% to 20% of patients achieve a complete response after conventional induction treatment. Attempts at improving the progression-free survival and overall survival by administering repeated (tandem) HDM have generally failed to overcome poor prognostic factors, such as an unfavorable karyotype or high serum β-2 microglobulin.2
Since the introduction of novel agents like thalidomide, bortezomib, and lenalidomide during remission-induction treatment before HDM, the response rates and depth of response have improved significantly both before and after transplantation. Recently, the results of 2 randomized trials clearly illustrated the significant advantage of adding bortezomib to dexamethasone, as done by the French IFM group, or thalidomide to dexamethasone and doxorubicin, as done by the Dutch HOVON group.3-5 Several other trials that were presented at recent meetings of the American Society of Hematology have demonstrated that the combination of bortezomib plus thalidomide (VTD) during induction or maintenance treatment is associated with high complete response rates before and after transplantation, resulting in significantly improved time to progression or progression-free survival6 (see figure).
All together, these and other trials point to the fact that effective and well-tolerated induction regimens using novel agents can be used in the outpatient setting and that these may have a bigger impact on progression-free survival than can be achieved with tandem HDM. Even the dose of melphalan may be less critical than we had thought previously. The current Italian study failed to demonstrate a survival advantage for HDM200 over HDM100, even though it was performed before the novel agents mentioned above became widely available. The recently published VISTA trial showed that conventional-dose melphalan, when combined with bortezomib, can achieve a complete response rate of 30%, which is comparable with or better than with vincristin, adriamycin, dexamethasone (VAD) followed by HDM.7 The challenge for the future therefore is no longer applying more force to our patients. Rather, our efforts should be aimed at finding the optimal combination of conventional and novel agents during induction treatment to increase the complete remission (CR) rate, including applying stringent polyclonal CR before and after administering HDM. Additional benefit for the patient may come from postintensification or consolidation therapy or maintenance treatment with nontoxic regimens.
Achieving sustained CR is a major goal for future investigations. Results from recent trials indicate that thalidomide during maintenance may prolong progression-free survival, although as yet not overall survival.5,8 It may well be that subgroup analyses of large prospective trials may provide insight to which patients benefit from the use of novel agents in specific settings.9 The trial performed by the Italian group may provide the background for omitting the paradigm of HDM200 as the absolute standard and opening up new ways to investigate combinations of novel agents with standard-dose cytostatics as an alternative postremission induction treatment in transplantation for myeloma.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■