Lymphopenia is thought to be a major cause of tolerance breakdown. In a lymphopenic environment, self-recognition events induce some T cells to expand strongly (a mechanism known as spontaneous proliferation). In this study, we show that in C57BL/6 mice, the repertoire resulting from lymphopenia-induced spontaneous CD4+ T-cell proliferation included a proportion of regulatory T cells as large as that observed in a normal mouse, and no autoimmune disorder was observed. By contrast, in nonobese diabetic mice, differences in the ability of conventional and regulatory T cells to expand in response to lymphopenia led to an unbalance between these 2 T-cell compartments at the expense of regulatory T cells, resulting in the onset of autoimmune diseases. Notably, this accounted for the rapid transfer of diabetes with small numbers of BDC2.5 CD4+ T cells. Thus, lymphopenia does not itself induce autoimmunity, but it should be considered as a cofactor for the development of autoimmune disorders.
Introduction
During thymic differentiation, the recognition of self-peptide/self–major histocompatibility complex (MHC) molecule ligands and the resulting activation process (positive selection) are required for the differentiation of immature thymocytes into fully mature lymphocytes.1,–3 In normal adult mice, although such self-recognition events in the periphery do not lead to the activation of the resulting naive T cells,4,5 such signals play a role in selecting and maintaining the naive T-cell repertoire.6,7
In a lymphopenic environment, self-recognition events and interleukin (IL)–7 induce peripheral T cells to proliferate.8 More precisely, it has been shown that peripheral CD4+ T cells can be subdivided into 2 subsets as a function of their behavior after transfer into lymphopenic mice.9,10 The first subset cycles slowly, and corresponds to the majority of injected T cells. This process was named homeostatic T-cell proliferation. This slow and limited proliferation of the bulk of the T cells results directly from the greater availability of IL-7 in lymphopenic environments and does not involve self-recognition.11,12 The second subset corresponds to a small proportion of the initially injected CD4+ T cells expanding strongly in response to interactions with self-peptides or commensal bacterium-derived peptides presented by MHC class II molecules.7,13 This process was recently given the name “spontaneous proliferation” by Min et al.12 Unlike homeostatic proliferation, spontaneous proliferation does not depend on IL-7 availability, but it does require interactions with self-ligands.
Several bacterial and viral infections induce lymphopenia of variable severity, ranging from the profound CD4+ T-cell deficiency caused by human immunodeficiency virus to milder, transient lymphopenia. Severe lymphopenia is also observed after chemo- or radiotherapy. T-cell lymphopenia may also develop with age, due in part to thymic involution. All of these situations are correlated with a higher frequency of onset of autoimmune disorders in humans.14,15 Similarly, many studies in mouse models have suggested that lymphopenia-induced proliferation may contribute to the onset of inflammatory and autoimmune diseases such as gastritis,16,17 inflammatory bowel disease,18 rheumatoid arthritis,19 experimental autoimmune encephalomyelitis,20 and type 1 diabetes.21,22
Lymphopenia may therefore be a major cause of immune tolerance breakdown. By inducing the spontaneous proliferation of residual T cells, lymphopenia may result in repertoire alterations, with the overrepresentation of activated autoreactive effectors. This increase might result in an unbalance between the regulatory and conventional T-cell compartments, triggering the development of autoimmune disorders.
We tested this hypothesis, by determining the precise frequency and nature of CD4+ T cells undergoing spontaneous proliferation in a lymphopenic environment in limiting dilution analyses in vivo. We conducted these experiments in parallel in a conventional mouse strain (C57BL/6 mice) and in the nonobese diabetic (NOD) mouse strain, a well-known model of type 1 diabetes.
Methods
Mice
C57BL/6 mice (CD45.2) were obtained from Charles River Laboratories. CD45.1 C57BL/6 mice, C57BL/6 CD3ϵ−/− mice, NOD mice (CD45.1), CD45.2 NOD mice, NOD Cα−/− mice (CD45.1), BDC2.5 NOD mice, and NOD severe combined immunodeficiency (SCID) mice were maintained in our own animal facilities, under specific pathogen-free conditions. C57BL/6 CD3ϵ−/− mice were crossed with MHC IIΔ/Δ mice to obtain CD3ϵ/MHC II double-deficient mice (CD3ϵ−/− IIKO mice). CD45.2 C57BL/6 Cα−/− mice were provided by Dr Agnès Lehuen (Inserm), and C57BL/6 IL-2−/− mice by Dr Antonio Freitas (Institut Pasteur, Paris, France). Six-week-old male C57BL/6 or NOD mice were used as donor mice; 4- to 10-week-old mice were used as recipient mice. All donor NOD mice were nondiabetic. Experiments were carried out in accordance with the guidelines of the French Veterinary Department.
Cell suspensions
Peripheral and mesenteric lymph nodes (LNs) and spleen tissue were homogenized and passed through a nylon cell strainer (BD Falcon) in RPMI 1640 Glutamax (Gibco) supplemented with 10% fetal calf serum (FCS; Biochrom) for adoptive transfer (LNs only), or in 5% FCS, 0.1% NaN3 (Sigma-Aldrich) in phosphate-buffered saline, for flow cytometry (pooled LN and spleen cells = periphery).
Adoptive transfer of CD4+ and CD8+ T cells
LN cells (pooled superficial cervical, axillary, brachial, inguinal, and mesenteric LNs) were incubated on ice for 20 minutes with a mixture of anti-CD8 (53-6.7) or anti-CD4 (GK1.5) antibody with anti-CD11b (Mac-1), anti-GR1 (8C5), and anti-CD19 (1D3) antibodies, obtained from hybridoma supernatants, and then with magnetic beads coupled to anti-rat immunoglobulins (Dynal Biotech). Purified T-cell subsets were generally 97% to 99% pure. CD4+CD25+ T cells were isolated by magnetic-activated cell sorting (Miltenyi Biotec). Briefly, CD25+ and CD25− T cells were isolated from purified CD4+ T cells by incubation with biotinylated anti-CD25 antibody (PC61) and antibiotin microbeads. In some experiments (Figure 4), purified CD4+ T cells were labeled with fluorescein isothiocyanate anti-CD44 (1M7) and phycoerythrin anti-CD25 (PC61), and CD4+CD25−CD44−/low and CD4+CD25−CD44high T cells were then purified by sorting in a FACSAria flow cytometer (BD Biosciences). Purified CD4+ T cells (10 cells to 5 × 106 cells) were injected intravenously into sex-matched lymphopenic recipient mice. Where indicated, CD4+ T cells were labeled with 5 μM 5,6-carboxyfluorescein diacetate-succinimyl ester (CFSE; Molecular Probes) before injection. In some experiments, 5 × 104 purified CD8+ T cells were coinjected with purified CD4+ T cells. Seven days to 3 months after transfer, the presence or absence of CD4+ T cells was determined in the periphery (pooled spleen and superficial cervical, axillary, brachial, inguinal, and mesenteric LNs) of each recipient mouse.
Adoptive transfer of diabetes
CD4+ and CD4+CD25− T cells from the pooled LNs and spleens of 12- to 14-week-old BDC2.5 NOD mice were injected into NOD SCID recipients. Mice were tested every other day for glycosuria and glycemia. Mice with blood glucose levels exceeding 300 mg/dL were classified as overtly diabetic. Pancreatic LNs were harvested on the day of diabetes diagnosis, or at the end of the experiment for mice that remained healthy, and Foxp3 expression by T cells was analyzed.
Cell surface staining and flow cytometry
Cell suspensions were collected and dispensed into 96-well round-bottom microtiter plates (Greiner Bioscience; 6 × 106 cells/well). Surface staining was performed by incubating the cells on ice, for 15 minutes per step, with antibodies in 5% FCS (Biochrom), 0.1% NaN3 (Sigma-Aldrich) in phosphate-buffered saline. Each cell-staining reaction was preceded by a 15-minute incubation with purified anti-CD16/32 antibodies (FcγRII/III block, 2.4G2) obtained from hybridoma supernatants. Peridin chlorophyll protein-conjugated anti-CD4 (RM4-5), fluorescein isothiocyanate–conjugated anti-CD8α (53-6.7), phycoerythrin-conjugated anti–T-cell receptor (TCR)β (H57-597), anti-CD25 (PC61), anti-CD44 (1M781), anti-Vβ4 (KT4), and anti-Vβ8 (F23.1) antibodies, phycoerythrin cyanin 7–conjugated anti-CD3 (145-2C11), biotinylated anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD25 (PC61), anti-CD44 (1M7), anti-CD69 (H1.2F3), anti-CD45RB (16A), and allophycocyanin-conjugated streptavidin were obtained from BD Biosciences. Allophycocyanin Alexa Fluor 750–conjugated anti-CD8 (53-6.7) was obtained from eBioscience. For intranuclear Foxp3 staining, cells were first fixed and permeabilized with the eBioscience Foxp3 staining buffer set, and then stained with phycoerythrin-conjugated anti-Foxp3 antibody (FJK-16s). Four-color and 6-color immunofluorescence analyses were carried out with a FACSCalibur flow cytometer and a BDLSRII flow cytometer, respectively (BD Biosciences). List-mode data files were analyzed with CellQuest software (BD Biosciences).
Histology
Organs were fixed in 8.6% formaldehyde and 4.8% acetic acid in distilled water. They were then embedded in paraffin, and 3-μm sections were cut and stained with hematoxylin and eosin.
Statistics
Limiting dilution frequencies were calculated with L-Calc software (StemCell Technologies). Results are expressed as mean frequencies. The statistical accuracy of the estimates was determined by χ2 analysis, generating 95% confidence intervals. Data are expressed as means plus or minus SEM, and the significance of differences between 2 means was assessed with the nonparametric Mann-Whitney test or with a parametric 2-tailed unpaired Student t test when populations of values follow the normal distribution (Figures 3B, 5B, and 7B). Differences were considered statistically significant when P value was less than .05. Kaplan-Meier estimation was used to calculate the incidence of diabetes, and the log-rank test was used to evaluate significance.
Results
Frequency of CD4+ T cells undergoing strong expansion in response to lymphopenia in C57BL/6 mice
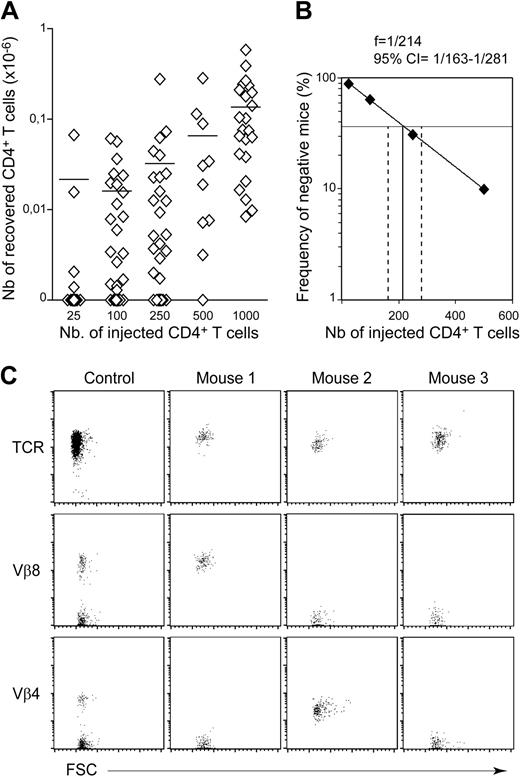
Our study was first conducted in C57BL/6 mice, a conventional mouse strain that does not naturally develop autoimmune disorders. We determined the precise frequency of CD4+ T cells undergoing spontaneous proliferation in a lymphopenic environment in limiting dilution analyses in vivo. CD4+ T cells were found in all recipient mice (C57BL/6 CD3ϵ−/− mice) receiving an initial injection of at least 103 CD4+ T cells (Figure 1A; Table 1). Notably, a mean of 1.36 × 105 CD4+ T cells was recovered from the periphery of mice injected with 103 CD4+ T cells. Thus, the injected cells increased in number by a factor of at least 100. CD4+ T cells were found in only some of the mice receiving less than 103 CD4+ T cells, confirming that only part of the peripheral CD4+ T-cell population was able to expand strongly in a lymphopenic environment. For example, CD4+ T cells were found in only 19 of 53 recipient mice injected with 102 CD4+ T cells, indicating that more than half the recipient mice had not received a single CD4+ T lymphocyte able to undergo spontaneous proliferation in a lymphopenic environment. This proportion remained unchanged for at least 3 months (Table 1). Thus, a 1-month period was considered sufficient to visualize all CD4+ T-cell clones undergoing strong expansion in response to lymphopenia. The proportion of negative recipients (ie, mice that received injections, but in which no CD4+ T cells were detected) was determined and plotted as a function of the absolute number of CD4+ T cells injected (Figure 1B). Limiting dilution analysis showed that, in the periphery of C57BL/6 mice, 1 CD4+ T cell in every 214 (95% confidence interval = 1/163-1/281) expanded strongly in a lymphopenic environment. This frequency suggests that most of the CD4+ T cells recovered from the periphery of a recipient mouse were derived from the strong expansion of a single CD4+ T cell, in situations in which small numbers of CD4+ T cells (up to 250 CD4+ T cells) were injected. Accordingly, the absolute number of CD4+ T cells recovered did not differ significantly between positive recipient mice initially injected with 25, 100, or 250 CD4+ T cells. Furthermore, when 102 CD4+ T cells were injected into the mice, all the CD4+ T cells recovered from a single mouse expressed a single TCR Vβ chain (Figure 1C).
Frequency of CD4+ T cells undergoing spontaneous proliferation in lymphopenic C57BL/6 mice. Twenty-five to 1000 CD4+ T cells from pooled superficial cervical, axillary, brachial, inguinal, and mesenteric LNs of CD45.1 C57BL/6 mice were injected into CD45.2 C57BL/6 CD3ϵ−/− mice. One month after transfer, the presence of CD45.1+CD4+CD8− TCR+ cells in the periphery (pooled LN and spleen cells) of recipient mice was individually tested. (A) Absolute numbers of recovered CD45.1+CD4+CD8− TCR+ cells are shown as a function of the number of injected CD4+ T cells. Each point represents an individual mouse, and horizontal lines represent the mean number of CD45.1+CD4+CD8− TCR+ cells recovered from positive mice. (B) Numbers of injected CD4+ T cells are plotted against the log frequency of negative mice (ie, injected mice in which CD4+ T cells were not detected 1 month after transfer). The frequency of C57BL/6 autoreactive CD4+ T cells was estimated at 1/214 (95% confidence limits, 1/163 to 1/281). (C) TCR/forward light scatter (FSC), Vβ8/FSC, and Vβ4/FSC dot plots for gated CD45.1+CD4+CD8− T cells are shown for 1 control C57BL/6 mouse and 3 representative recipient mice injected with 100 CD4+ T cells.
Frequency of CD4+ T cells undergoing spontaneous proliferation in lymphopenic C57BL/6 mice. Twenty-five to 1000 CD4+ T cells from pooled superficial cervical, axillary, brachial, inguinal, and mesenteric LNs of CD45.1 C57BL/6 mice were injected into CD45.2 C57BL/6 CD3ϵ−/− mice. One month after transfer, the presence of CD45.1+CD4+CD8− TCR+ cells in the periphery (pooled LN and spleen cells) of recipient mice was individually tested. (A) Absolute numbers of recovered CD45.1+CD4+CD8− TCR+ cells are shown as a function of the number of injected CD4+ T cells. Each point represents an individual mouse, and horizontal lines represent the mean number of CD45.1+CD4+CD8− TCR+ cells recovered from positive mice. (B) Numbers of injected CD4+ T cells are plotted against the log frequency of negative mice (ie, injected mice in which CD4+ T cells were not detected 1 month after transfer). The frequency of C57BL/6 autoreactive CD4+ T cells was estimated at 1/214 (95% confidence limits, 1/163 to 1/281). (C) TCR/forward light scatter (FSC), Vβ8/FSC, and Vβ4/FSC dot plots for gated CD45.1+CD4+CD8− T cells are shown for 1 control C57BL/6 mouse and 3 representative recipient mice injected with 100 CD4+ T cells.
Regulatory CD4+ T cells need help from conventional CD4+ T cells to undergo strong expansion in response to lymphopenia
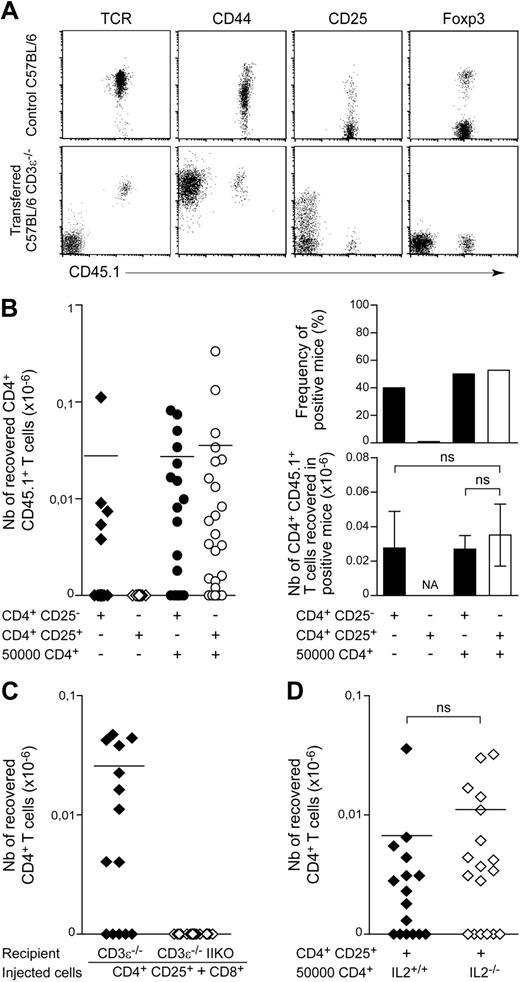
We then characterized the CD4+ T cells recovered from the periphery of positive recipients initially injected with 102 CD4+ T lymphocytes. Consistent with most previous reports on lymphopenia-induced proliferation,23,,–26 the recovered CD4+ T cells had a memory-like phenotype, with high surface CD44 levels (Figure 2A) and low CD45RB levels (data not shown). Surprisingly, we found no CD4+ T cells expressing Foxp3 in any of the positive recipient mice tested. Consistent with these findings, the CD4+ T cells recovered expressed CD25 only weakly, if at all, at the cell surface (Figure 2A).
The spontaneous proliferation of regulatory T cells in a lymphopenic environment requires help from conventional CD4+ T cells. (A) A total of 102 LN CD4+ T cells from CD45.1 C57BL/6 mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice. Fluorescence dot plots show TCR, CD44, CD25, and Foxp3 expression on gated CD4+CD8− T cells from a control C57BL/6 mouse and a representative recipient mouse 1 month after transfer. (B) A total of 102 CD4+CD25− or CD4+CD25+ T cells from CD45.1 C57BL/6 mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice, alone or together with 5 × 104 CD45.2+CD4+ T cells. One month later, the presence of CD45.1+CD4+CD8− TCR+ cells in the periphery of recipient mice was individually tested. Left: absolute numbers of CD45.1+CD4+CD8− TCR+ cells; right: frequency of positive mice and absolute numbers of CD45.1+CD4+CD8− TCR+ cells in positive mice. Data show means ± SEM of mice for 3 independent experiments. (C) A total of 102 CD4+CD25+ T cells from CD45.1 C57BL/6 mice and 5 × 104 CD8+ T cells from CD45.2 C57BL/6 mice was cotransferred into C57BL/6 CD3ϵ−/− IIKO or C57BL/6 CD3ϵ−/− mice. The absolute numbers of CD45.1+CD4+CD8− TCR+ cells recovered from recipient mice 1 month after transfer are shown. (D) A total of 102 CD45.1+CD4+CD25+ T cells from C57BL/6 mice was coinjected with 5 × 104 CD4+ T cells from C57BL/6 IL-2−/− mice or C57BL/6 control littermates into C57BL/6 CD3ϵ−/− mice. Absolute numbers of CD45.1+CD4+CD8− TCR+ cells recovered from recipient mice 1 month after transfer are shown. Each point represents an individual mouse, and horizontal lines represent the mean number of CD45.1+CD4+CD8− TCR+ cells in positive mice (B left; C-D). ns, not significant; NA, not applicable.
The spontaneous proliferation of regulatory T cells in a lymphopenic environment requires help from conventional CD4+ T cells. (A) A total of 102 LN CD4+ T cells from CD45.1 C57BL/6 mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice. Fluorescence dot plots show TCR, CD44, CD25, and Foxp3 expression on gated CD4+CD8− T cells from a control C57BL/6 mouse and a representative recipient mouse 1 month after transfer. (B) A total of 102 CD4+CD25− or CD4+CD25+ T cells from CD45.1 C57BL/6 mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice, alone or together with 5 × 104 CD45.2+CD4+ T cells. One month later, the presence of CD45.1+CD4+CD8− TCR+ cells in the periphery of recipient mice was individually tested. Left: absolute numbers of CD45.1+CD4+CD8− TCR+ cells; right: frequency of positive mice and absolute numbers of CD45.1+CD4+CD8− TCR+ cells in positive mice. Data show means ± SEM of mice for 3 independent experiments. (C) A total of 102 CD4+CD25+ T cells from CD45.1 C57BL/6 mice and 5 × 104 CD8+ T cells from CD45.2 C57BL/6 mice was cotransferred into C57BL/6 CD3ϵ−/− IIKO or C57BL/6 CD3ϵ−/− mice. The absolute numbers of CD45.1+CD4+CD8− TCR+ cells recovered from recipient mice 1 month after transfer are shown. (D) A total of 102 CD45.1+CD4+CD25+ T cells from C57BL/6 mice was coinjected with 5 × 104 CD4+ T cells from C57BL/6 IL-2−/− mice or C57BL/6 control littermates into C57BL/6 CD3ϵ−/− mice. Absolute numbers of CD45.1+CD4+CD8− TCR+ cells recovered from recipient mice 1 month after transfer are shown. Each point represents an individual mouse, and horizontal lines represent the mean number of CD45.1+CD4+CD8− TCR+ cells in positive mice (B left; C-D). ns, not significant; NA, not applicable.
We then investigated whether assistance from conventional T cells was required for regulatory CD4+ T-cell expansion in a lymphopenic environment. One month after transfer, no CD4+ T cells were found in mice injected with 102 CD4+CD25+ T cells alone (Figure 2B). By contrast, if 5 × 104 CD45.2+CD4+ T cells were injected together with 102 CD45.1+CD4+CD25+ T cells, this latter subset of cells expanded in response to lymphopenia. The recovered CD45.1+CD4+ T cells also expressed CD25 and Foxp3 (data not shown). The observed frequency of positive recipient mice and the mean absolute number of T cells in positive recipient mice were not significantly different from those obtained after injection of CD4+CD25− T cells alone or together with helper CD4+ T cells (Figure 2B). Thus, regulatory CD4+ T cells require help from conventional CD4+ T cells to expand in response to lymphopenia.
Strong expansion of regulatory CD4+ T cells in response to lymphopenia requires interactions with self, but not IL-2 production by conventional T cells
We investigated whether strong expansion of regulatory CD4+ T cells in response to lymphopenia required interactions with MHC class II molecules, as already demonstrated for the bulk of peripheral CD4+ T cells,7 by injecting 102 peripheral CD4+CD25+ T cells together with CD8+ T cells into C57BL/6 CD3ϵ−/− mice lacking or not lacking MHC class II molecule expression (Figure 2C). One month after transfer, the expansion of CD4+CD25+ T cells was detected only in recipient mice expressing MHC class II molecules. Thus, like conventional CD4+ T cells, CD8+ T cells can provide the help required for regulatory CD4+ T cells to expand in response to lymphopenia; this expansion results from interactions with self-MHC class II molecules.
We then investigated whether IL-2 production was the help provided by conventional CD4+ T cells for regulatory CD4+ T-cell expansion in a lymphopenic environment. We injected 102 peripheral CD4+CD25+ T cells, together with CD4+ T cells from wild-type or IL-2-deficient mice, into C57BL/6 CD3ϵ−/− mice. No significant difference was observed between the 2 groups (Figure 2D). Thus, IL-2 production by conventional CD4+ T cells was not essential for the expansion of the regulatory CD4+ T-cell population in response to lymphopenia.
The frequency of regulatory T cells among the CD4+ T cells recovered after spontaneous proliferation in a lymphopenic environment varies with the number of CD4+ T cells injected
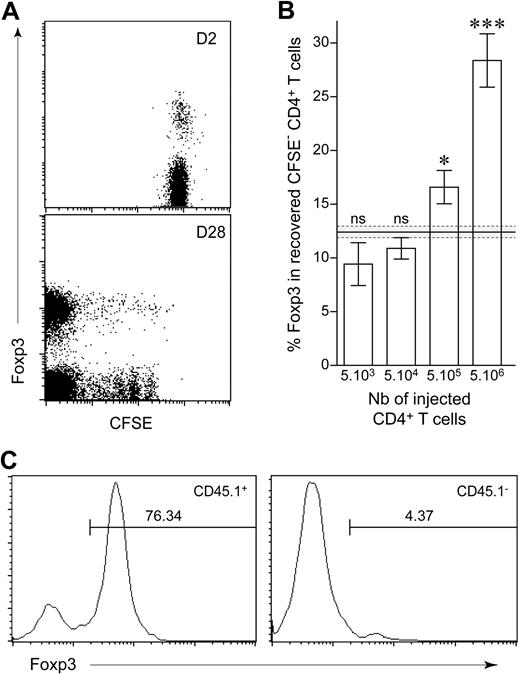
We injected various numbers of peripheral CD4+ T cells (from 5 × 103 to 5 × 106 cells) into CD3ϵ−/− mice to determine whether the extent of initial lymphopenia affected the balance between conventional and regulatory T-cell compartments. We distinguished between slowly and strongly proliferating CD4+ T cells, by labeling purified lymphocytes with CFSE before transfer and restricting the analysis to CD4+ T cells that had completely diluted this intracytoplasmic dye 1 month after transfer (Figure 3A). The injection of larger numbers of CD4+ T cells resulted in a larger proportion of regulatory CD4+ T cells among CFSE−CD4+ T cells (Figure 3B). This increase in the proportion of regulatory CD4+ T cells induced by spontaneous proliferation was not due to the conversion of conventional CD4+ T cells into regulatory T cells with the induction of Foxp3 expression. Indeed, after the injection of CFSE-labeled CD25+CD4+ T cells together with CD25−CD4+ T cells, Foxp3 expression by CFSE− CD4+ T cells 1 month after transfer was mostly restricted to the CD25+ T cells initially injected (Figure 3C). Thus, the more CD4+ T cells were initially injected, the more regulatory CD4+ T-cell expansion was favored. This suggests that the injection of a large number of conventional CD4+ T cells results in more help received by regulatory CD4+ T cells.
Frequency of regulatory CD4+ T cells recovered after spontaneous proliferation in a lymphopenic environment. (A) A total of 5 × 106 LN CD4+ T cells from CD45.1 C57BL/6 mice was purified, labeled with CFSE, and transferred into C57BL/6 CD3ϵ−/− hosts. Two and 28 days after transfer, pooled LNs and spleen were analyzed for Foxp3 expression. Fluorescence Foxp3/CFSE dot plots are shown for CD45.1+ CD4+ T cells 2 and 28 days after transfer. (B) A total of 5 × 103 to 5 × 106 CFSE-labeled T cells from CD45.1 C57BL/6 mice was transferred into C57BL/6 CD3ϵ−/− mice and analyzed 1 month after transfer. Proportion of CD45.1+ CFSE− CD4+ T cells expressing Foxp3 is shown as means ± SEM for 3 independent experiments. The gray area indicates the mean ± SEM proportion of Foxp3-expressing cells among peripheral CD4+ T cells from control C57BL/6 mice. ***P < .001; *P < .05; ns, not significant. (C) A total of 106 CD4+CD25+ T cells from CD45.1 C57BL/6 mice and 106 CD4+CD25− T cells from CD45.2 C57BL/6 mice was labeled with CFSE and coinjected into C57BL/6 CD3ϵ−/− mice. Representative Foxp3 fluorescence histograms of gated CD45.1+ or CD45.1− CFSE− CD4+ T cells 1 month after transfer. Percentages of Foxp3+ cells are indicated.
Frequency of regulatory CD4+ T cells recovered after spontaneous proliferation in a lymphopenic environment. (A) A total of 5 × 106 LN CD4+ T cells from CD45.1 C57BL/6 mice was purified, labeled with CFSE, and transferred into C57BL/6 CD3ϵ−/− hosts. Two and 28 days after transfer, pooled LNs and spleen were analyzed for Foxp3 expression. Fluorescence Foxp3/CFSE dot plots are shown for CD45.1+ CD4+ T cells 2 and 28 days after transfer. (B) A total of 5 × 103 to 5 × 106 CFSE-labeled T cells from CD45.1 C57BL/6 mice was transferred into C57BL/6 CD3ϵ−/− mice and analyzed 1 month after transfer. Proportion of CD45.1+ CFSE− CD4+ T cells expressing Foxp3 is shown as means ± SEM for 3 independent experiments. The gray area indicates the mean ± SEM proportion of Foxp3-expressing cells among peripheral CD4+ T cells from control C57BL/6 mice. ***P < .001; *P < .05; ns, not significant. (C) A total of 106 CD4+CD25+ T cells from CD45.1 C57BL/6 mice and 106 CD4+CD25− T cells from CD45.2 C57BL/6 mice was labeled with CFSE and coinjected into C57BL/6 CD3ϵ−/− mice. Representative Foxp3 fluorescence histograms of gated CD45.1+ or CD45.1− CFSE− CD4+ T cells 1 month after transfer. Percentages of Foxp3+ cells are indicated.
When low numbers of CD4+ T cells were injected (5 × 104), the proportion of Foxp3+ cells among CFSE− CD4+ T cells increased with time from 4.3% (7 days after transfer) to 16.4% (28 days after transfer; P = .03), suggesting that expansion of conventional CD4+ T cells occurred first and that only when they have reached a sufficient number, they can provide the help required for regulatory CD4+ T-cell expansion in response to lymphopenia (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The frequency of peripheral CD4+ T cells undergoing spontaneous proliferation in a lymphopenic environment is higher in NOD mice than in C57BL/6 mice
We then pursued our studies in the NOD mouse strain, a well-known model of type 1 diabetes. Surprisingly, after the transfer of 25 CD4+ T cells, expansion was observed in almost two-thirds of recipient mice (Figure 4A), whereas, with a similar input, only approximately 10% of recipient mice of the C57BL/6 background were found positive (Tables 1–2). Limiting dilution analyses showed that, in the periphery of NOD mice, approximately 1 CD4+ T cell in 25 was able to expand strongly in a lymphopenic environment, this frequency being 8 times higher than that in C57BL/6 mice. Moreover, if very small numbers of T cells were injected, such that the CD4+ T cells recovered originated from a single CD4+ T lymphocyte, then expansion was greater in the NOD than in the C57BL/6 background (Tables 1–2). Indeed, approximately 0.11 × 106 CD4+ T cells were recovered from the periphery of NOD recipient mice injected with 25 CD4+ T cells, whereas less than 0.02 × 106 T lymphocytes were found in C57BL/6 recipient mice injected with 4 times as many cells (P = .004). Accordingly, when large numbers of CD4+ T cells were injected into the mice, far more CD4+ T cells were found in NOD than in C57BL/6 mice (Figure 4A).
Increased frequency of peripheral CD4+ T cells undergoing spontaneous proliferation in NOD mice compared with C57BL/6 mice. (A) A total of 10 to 5 × 104 LN CD4+ T cells from CD45.2 NOD mice was transferred into CD45.1 NOD Cα−/− mice. A total of 25 to 5 × 104 CD4+ T cells from CD45.1 C57BL/6 mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice. Absolute numbers of CD45.2+CD4+CD8− TCR+ cells in NOD Cα−/− recipient mice (circle) and of CD45.1+CD4+CD8− TCR+ cells in C57BL/6 CD3ϵ−/− recipient mice (diamond) are shown. (B) Absolute numbers of CD45.1+CD4+CD8− TCR+ cells in C57BL/6 CD3ϵ−/− mice or C57BL/6 Cα−/− mice receiving 5 × 103 CD4+ T cells. (C) CD25, CD44, CD45RB, and CD69 expression as a function of Foxp3 expression on gated LN CD4+CD8− T cells was compared between C57BL/6 and NOD mice. The dot plots shown were generated from the data for 1 mouse, but are representative of 3 individual experiments with at least 2 mice per group. (D) LN CD4+ T cells from C57BL/6 and NOD mice were purified, and naive CD4+ T cells were electronically sorted on the basis of their nonexpression of CD25 and their low or absent expression of CD44. A total of 5 × 103 purified naive CD4+ T cells (CD4+CD25−CD44−/low) from CD45.1 C57BL/6 mice and CD45.2 NOD mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice and CD45.1 NOD Cα−/− mice, respectively. Absolute numbers of recovered CD45.1+CD4+CD8− TCR+ cells in C57BL/6 CD3ϵ−/− recipient mice and of CD45.2+CD4+CD8− TCR+ cells in NOD Cα−/− recipient mice are shown 1 month after transfer. ***P < .001; **P < .01; ns, not significant. Each point represents an individual mouse, and horizontal lines represent the mean number of CD4+ T cells recovered from positive mice (A-B, D).
Increased frequency of peripheral CD4+ T cells undergoing spontaneous proliferation in NOD mice compared with C57BL/6 mice. (A) A total of 10 to 5 × 104 LN CD4+ T cells from CD45.2 NOD mice was transferred into CD45.1 NOD Cα−/− mice. A total of 25 to 5 × 104 CD4+ T cells from CD45.1 C57BL/6 mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice. Absolute numbers of CD45.2+CD4+CD8− TCR+ cells in NOD Cα−/− recipient mice (circle) and of CD45.1+CD4+CD8− TCR+ cells in C57BL/6 CD3ϵ−/− recipient mice (diamond) are shown. (B) Absolute numbers of CD45.1+CD4+CD8− TCR+ cells in C57BL/6 CD3ϵ−/− mice or C57BL/6 Cα−/− mice receiving 5 × 103 CD4+ T cells. (C) CD25, CD44, CD45RB, and CD69 expression as a function of Foxp3 expression on gated LN CD4+CD8− T cells was compared between C57BL/6 and NOD mice. The dot plots shown were generated from the data for 1 mouse, but are representative of 3 individual experiments with at least 2 mice per group. (D) LN CD4+ T cells from C57BL/6 and NOD mice were purified, and naive CD4+ T cells were electronically sorted on the basis of their nonexpression of CD25 and their low or absent expression of CD44. A total of 5 × 103 purified naive CD4+ T cells (CD4+CD25−CD44−/low) from CD45.1 C57BL/6 mice and CD45.2 NOD mice was injected into CD45.2 C57BL/6 CD3ϵ−/− mice and CD45.1 NOD Cα−/− mice, respectively. Absolute numbers of recovered CD45.1+CD4+CD8− TCR+ cells in C57BL/6 CD3ϵ−/− recipient mice and of CD45.2+CD4+CD8− TCR+ cells in NOD Cα−/− recipient mice are shown 1 month after transfer. ***P < .001; **P < .01; ns, not significant. Each point represents an individual mouse, and horizontal lines represent the mean number of CD4+ T cells recovered from positive mice (A-B, D).
The above experiments were performed in the C57BL/6 background with CD3ϵ−/− mice and in the NOD mice, with Cα−/− mice as lymphopenic recipients. We checked that this did not account for the discrepancies observed between the 2 mouse strains, by injecting 5 × 103 CD4+ T cells from C57BL/6 mice into C57BL/6 CD3ϵ−/− mice and C57BL6 Cα−/− mice in parallel. One month after transfer, donor cell recovery was similar in the 2 recipient mouse strains (Figure 4B).
The higher numbers of CD4+ T cells found in NOD recipient mice than in C57BL/6 recipient mice may derive from an increased frequency of activated/memory cells in the initial input of LN CD4+ T cells from NOD mice, compared with C57BL/6 mice. First, donor mice were 6-week-old male mice, thus well before the age of diabetes onset. Second, we analyzed and compared the phenotype of injected LN CD4+ T cells from both mouse strains, and no evidence for an increased frequency of activated/memory CD4+ T cells was found in NOD mice compared with C57BL/6 mice (Figure 4C). Third, we sorted naive CD4+ T cells on the basis of their nonexpression of CD25 and their nonexpression or low expression of CD44 (supplemental Figure 2) and injected them into lymphopenic recipient mice. Twenty-eight days later, far more CD4+ T cells were still found in the periphery of NOD recipient mice than in the periphery of C57BL/6 recipient mice (Figure 4D). Finally, when injected into lymphopenic recipients, we observed that purified activated/memory CD4+ T cells (CD25−/lowCD44highCD4+ T cells) did not expand to a greater extent than their naive (CD25−CD44−/lowCD4+ T cells) counterparts (supplemental Figure 2b). Altogether, these results clearly demonstrated that the differential ability of CD4+ T cells to expand in response to lymphopenia observed between NOD and C57BL/6 mice did not result from an increased proportion of activated/memory CD4+ T cells in the LN of NOD mice.
In NOD mice, the expansion of regulatory CD4+ T cells in response to lymphopenia is weaker than that of conventional CD4+ T cells
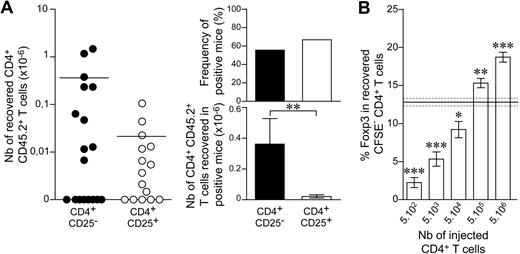
As observed in the C57BL/6 background, no Foxp3-expressing cells were recovered from the periphery of NOD recipient mice after the injection of 10 or 25 CD4+ T cells (data not shown). When we injected purified CD4+CD25+ T cells together with helper CD4+ T cells, positive recipient mice (mice in which cells derived from the CD4+CD25+ T cells initially injected were present 1 month after transfer) were found (Figure 5A), and the CD4+ T cells recovered from these recipient mice expressed Foxp3 (data not shown).
Frequencies of conventional and regulatory T cells undergoing spontaneous proliferation in lymphopenic NOD mice 1 month after reconstitution. (A) Twenty-five CD4+CD25− or CD4+CD25+ LN T cells from CD45.2 NOD mice and 2 × 103 CD4+ T cells from CD45.1 NOD mice were cotransferred into CD45.1 NOD Cα−/− recipients. Left: absolute numbers of recovered CD45.2+CD4+CD8− TCR+ cells. Each point represents an individual mouse, and horizontal lines represent the mean number of CD4+ T cells recovered from positive mice; right: frequency of positive mice and numbers of CD45.2+CD4+CD8− TCR+ cells recovered from positive mice. Data show means ± SEM from 3 independent experiments. (B) A total of 5 × 102 to 5 × 106 CD4+ T cells from CD45.2 NOD mice was labeled with CFSE and transferred into CD45.1 NOD Cα−/− mice. Proportion of CD45.2+ CFSE− CD4+ T cells expressing Foxp3. Results are from 2 independent experiments. The gray area represents the mean ± SEM proportion of Foxp3-expressing cells among CD4+ T cells from control NOD mice. ***P < .001; **P < .01; *P < .05.
Frequencies of conventional and regulatory T cells undergoing spontaneous proliferation in lymphopenic NOD mice 1 month after reconstitution. (A) Twenty-five CD4+CD25− or CD4+CD25+ LN T cells from CD45.2 NOD mice and 2 × 103 CD4+ T cells from CD45.1 NOD mice were cotransferred into CD45.1 NOD Cα−/− recipients. Left: absolute numbers of recovered CD45.2+CD4+CD8− TCR+ cells. Each point represents an individual mouse, and horizontal lines represent the mean number of CD4+ T cells recovered from positive mice; right: frequency of positive mice and numbers of CD45.2+CD4+CD8− TCR+ cells recovered from positive mice. Data show means ± SEM from 3 independent experiments. (B) A total of 5 × 102 to 5 × 106 CD4+ T cells from CD45.2 NOD mice was labeled with CFSE and transferred into CD45.1 NOD Cα−/− mice. Proportion of CD45.2+ CFSE− CD4+ T cells expressing Foxp3. Results are from 2 independent experiments. The gray area represents the mean ± SEM proportion of Foxp3-expressing cells among CD4+ T cells from control NOD mice. ***P < .001; **P < .01; *P < .05.
When help was provided, the proportions of positive recipient mice were similar after the injection of 25 CD25−CD4+ or 25 CD25+CD4+ T cells (Figure 5A). However, 1 month after transfer, absolute numbers of CD4+ T cells derived from the 25 CD4+ T cells initially injected were significantly higher for conventional (CD25−) than for regulatory (CD25+) CD4+ T cells (0.36 × 106 ± 0.16 and 0.022 × 106 ± 0.010, respectively; P = .009).
We then injected various numbers of CFSE-labeled CD4+ T cells into NOD Cα−/− mice. One month after transfer, we determined the frequency of Foxp3+ cells among the recovered CD4+ T cells (Figure 5B). As in the C57BL/6 background, the transfer of a large number of cells into NOD mice resulted in a proportion of the recovered CD4+ T cells being Foxp3+ higher than in the cells initially injected. However, in contrast to what was observed in C57BL/6 mice, after the injection of small numbers of CD4+ T cells (less than 5 × 105 CD4+ T cells), expansion in response to lymphopenia resulted in a significant decrease in the proportion of Foxp3-expressing cells (Figure 5B). This result did not derive from a lower proportion of regulatory T cells among LN CD4+ T cells in NOD donor mice compared with C57BL/6 donor mice. Indeed, in agreement with previously published observations,27,28 we found that, in the periphery of both mouse strains, a similar proportion of CD4+ T cells expressed Foxp3 (C57BL/6, 12.42 ± 0.53; NOD, 12.78 ± 0.51).
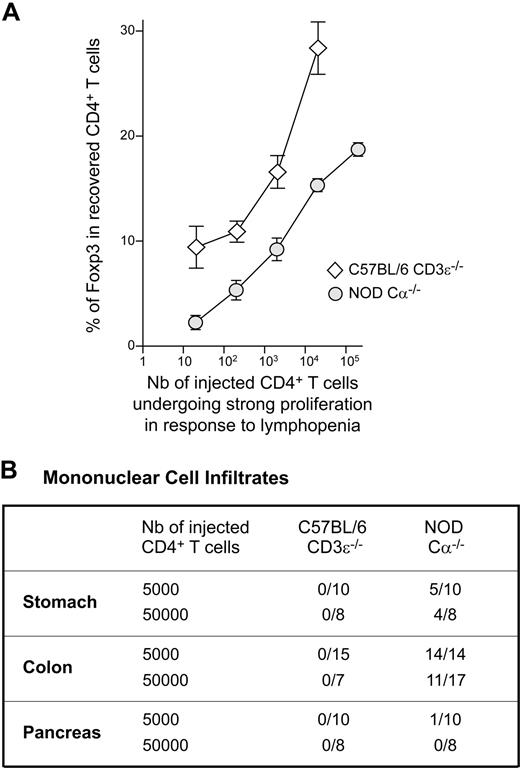
We rendered the results obtained for NOD and C57BL/6 mice more readily comparable (Figure 3B vs Figure 5B), by evaluating the number of initially injected CD4+ T cells able to expand strongly in response to lymphopenia by dividing the total number of CD4+ T cells injected into the mice by the frequency of responding cells, determined by limiting dilution analyses in vivo (1/214 for C57BL/6 mice versus 1/25 for NOD mice). The frequency of Foxp3-expressing cells among the recovered CD4+ T cells was plotted as a function of the calculated cell numbers (Figure 6A). Interestingly, the curve obtained for C57BL/6 mice was clearly above the curve obtained for NOD mice for all cell numbers. These data reflect the fact that, in NOD mice, at the single-cell level, conventional CD4+ T cells have a greater potential to expand strongly in response to lymphopenia than regulatory CD4+ T cells (Figure 5A).
Reduced proportion of regulatory CD4+ T cells and development of autoimmune disorders in reconstituted lymphopenic NOD mice. (A) A total of 5 × 102 to 5 × 106 CD4+ T cells from C57BL/6 and NOD mice was transferred into C57BL/6 CD3ϵ−/− and NOD Cα−/− mice, respectively. The number of initially injected CD4+ T cells expanding strongly in response to lymphopenia was evaluated by dividing the total number of CD4+ T cells injected into mice by the frequency of CD4+ T cells, as determined in limiting dilution analyses (1/214 for C57BL/6 mice, and 1/25 for NOD mice). The numbers obtained were plotted against the frequency of Foxp3+ cells among the CD4+ T cells recovered 1 month after transfer. (B) A total of 5 × 103 or 5 × 104 CD4+ T cells from C57BL/6 mice and from NOD mice was transferred into C57BL/6 CD3ϵ−/− mice and NOD Cα−/− mice, respectively. One month after transfer, mice were killed and paraffin-embedded sections of various organs were stained with hematoxylin and eosin. The presence of mononuclear cell infiltrates in the indicated organs is recorded. Results are expressed as the number of infiltrated organs among the total number of organs examined.
Reduced proportion of regulatory CD4+ T cells and development of autoimmune disorders in reconstituted lymphopenic NOD mice. (A) A total of 5 × 102 to 5 × 106 CD4+ T cells from C57BL/6 and NOD mice was transferred into C57BL/6 CD3ϵ−/− and NOD Cα−/− mice, respectively. The number of initially injected CD4+ T cells expanding strongly in response to lymphopenia was evaluated by dividing the total number of CD4+ T cells injected into mice by the frequency of CD4+ T cells, as determined in limiting dilution analyses (1/214 for C57BL/6 mice, and 1/25 for NOD mice). The numbers obtained were plotted against the frequency of Foxp3+ cells among the CD4+ T cells recovered 1 month after transfer. (B) A total of 5 × 103 or 5 × 104 CD4+ T cells from C57BL/6 mice and from NOD mice was transferred into C57BL/6 CD3ϵ−/− mice and NOD Cα−/− mice, respectively. One month after transfer, mice were killed and paraffin-embedded sections of various organs were stained with hematoxylin and eosin. The presence of mononuclear cell infiltrates in the indicated organs is recorded. Results are expressed as the number of infiltrated organs among the total number of organs examined.
Autoimmunity in NOD mice is correlated with the degree of lymphopenia
In NOD mice, the spontaneous proliferation induced by the injection of small numbers of CD4+ T cells (< 5 × 105 cells) led to an imbalance between the conventional and regulatory T-cell compartments, with a significant decrease in the proportion of regulatory CD4+ T cells. Consistent with this finding, most stomach and colon samples from NOD Cα−/− mice reconstituted with 5 × 103 or 5 × 104 CD4+ T cells contained leukocyte infiltrates 4 weeks after transfer (Figure 6B). By contrast, all the tested organs of C57BL/6 CD3ϵ−/− mice injected with similar numbers of CD4+ T cells remained leukocyte free at this time point. A mononuclear cell infiltrate was found in the exocrine pancreas of 1 in 10 of the NOD recipient mice tested (the islets of Langerhans were normal).
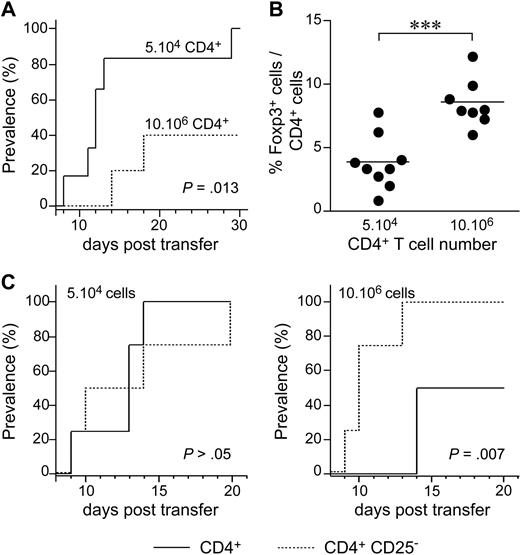
We then investigated the possible role of lymphopenia-induced proliferation in diabetes development, by injecting various numbers of CD4+ T cells from BDC2.5 TCR-transgenic NOD mice (BDC2.5 NOD mice) into NOD Cα−/− recipients. BDC2.5 NOD mice carry the rearranged TCR α and β chain genes from a diabetogenic, β-cell–specific, CD4+ T-cell clone isolated from a diabetic NOD mouse.29 When crossed with Cα- or recombination-activating gene (Rag)–deficient mice, BDC2.5 NOD mice rapidly developed diabetes. By contrast, wild-type BDC2.5 NOD mice were protected against diabetes. This may be accounted for by the generation of regulatory CD4+ T cells in wild-type mice (and not in BDC2.5 NOD Cα−/− or Rag−/− mice), conferring the observed protection.30,31 When 5 × 104 purified CD4+ T cells from BDC2.5 NOD mice were injected, all recipient mice rapidly developed diabetes (Figure 7A). By contrast, when 10 × 106 CD4+ T cells were injected, half the recipient mice remained healthy and the onset of diabetes was delayed in the other half. Protection was correlated with an increase in the proportion of regulatory T cells among CD4+ T cells in the pancreatic LN (Figure 7B). More precisely, after the injection of 5 × 104 CD4+ T cells, the proportion of CD4+ T cells that was Foxp3+ was lower than that after the transfer of 10 × 106 BDC2.5 CD4+ T cells. The depletion of CD25-expressing cells from the largest number of BDC2.5 CD4+ T cells (10 × 106 cells) administered abolished protection against the transfer of diabetes (Figure 7C), whereas no effect was observed after the depletion of these cells from the smallest number of BDC2.5 CD4+ T cells administered (5 × 104 cells).
The initial degree of host lymphopenia governs the expansion of regulatory T cells and, consequently, the development of adoptively transferred diabetes. (A) The prevalence of diabetes was determined in NOD SCID mice injected with 5 × 104 (plain line) or 10 × 106 (dotted line) CD4+ T cells from BDC2.5 NOD mice (n = 6). (B) Frequency of Foxp3-expressing cells among CD4+ T cells from the pancreatic LNs of NOD SCID mice injected with 5 × 104 or 10 × 106 BDC2.5 NOD CD4+ T cells. ***P < .001. (C) NOD SCID mice received 5 × 104 (left) or 10 × 106 (right) CD4+ or CD4+CD25− T cells from BDC2.5 NOD mice (n = 4/group).
The initial degree of host lymphopenia governs the expansion of regulatory T cells and, consequently, the development of adoptively transferred diabetes. (A) The prevalence of diabetes was determined in NOD SCID mice injected with 5 × 104 (plain line) or 10 × 106 (dotted line) CD4+ T cells from BDC2.5 NOD mice (n = 6). (B) Frequency of Foxp3-expressing cells among CD4+ T cells from the pancreatic LNs of NOD SCID mice injected with 5 × 104 or 10 × 106 BDC2.5 NOD CD4+ T cells. ***P < .001. (C) NOD SCID mice received 5 × 104 (left) or 10 × 106 (right) CD4+ or CD4+CD25− T cells from BDC2.5 NOD mice (n = 4/group).
Discussion
It has been reported that some peripheral CD4+ T cells undergo strong proliferation and expansion in response to lymphopenia.9,10 Nevertheless, the exact frequency and nature of the cells concerned remain unclear. We show in this study, by limiting dilution analysis in vivo, that these cells represent a minority of the peripheral CD4+ T-cell pool and that their proportion depends on the mouse strain studied. Indeed, whereas less than 0.5% of CD4+ T cells from C57BL/6 mice (1/214) expand strongly in a lymphopenic environment, approximately 4% of CD4+ T cells from NOD mice (1/25) undergo spontaneous proliferation in response to lymphopenia. We and others previously showed that spontaneous proliferation in response to lymphopenia is dependent on interactions with self-peptides or commensal bacterium-derived peptides presented by MHC class II molecules.7,13 Interestingly, previous studies have shown that thymic-negative selection is impaired in NOD mice.32,33 Thus, the greater ability of CD4+ T cells from NOD mice to undergo spontaneous proliferation may reflect a higher frequency of highly autoreactive T-cell clones migrating from the thymus to the periphery in NOD mice than in a conventional nonautoimmune disease-prone mouse strain (C57BL/6).
We and others have previously observed that in C57BL/6 mice, only 20% of CD4+ T cells transferred into lymphopenic mice efficiently migrated to peripheral lymphoid organs.7,26 We verified that homing of both conventional and regulatory CD4+ T cells to lymphoid organs after transfer did not differ between NOD and C57BL/6 mice (data not shown). If we take into account this parameter and correct our calculations according to this percentage, this would suggest that 5-fold more CD4+ T cells than what we calculated above would be able to undergo spontaneous proliferation in response to lymphopenia. Thus, approximately 1 CD4+ T cell in 5 would be able to expand strongly in a lymphopenic environment in NOD mice versus approximately 1 of 40 CD4+ T cells in C57BL/6 mice. Nevertheless, the engraftment of highly autoreactive T-cell clones may possibly be more efficient than that of the bulk of peripheral CD4+ T cells.
In both mouse strains, regulatory CD4+ T cells required help from conventional T cells to expand strongly in response to lymphopenia. It remains unclear whether this help allows regulatory T cells to proliferate or their progeny to survive. We cannot completely exclude either that conventional T cells could partially act by increasing the engraftment of regulatory CD4+ T cells. IL-2 allows regulatory CD4+ T cells to proliferate in vitro in response to anti-CD3 antibody,34,35 and has been shown to be crucial for peripheral regulatory CD4+ T-cell survival in vivo.36,–38 Moreover, Almeida et al have suggested that the extent of regulatory T-cell expansion during reconstitution of the peripheral T-cell compartment is linked to the number of conventional CD4+ T cells producing IL-2.39 Finally, lymphopenic people receiving IL-2 display a markedly expanded regulatory CD4+ T-cell compartment.40 All these data suggest that IL-2 production by conventional T cells provides the help required by regulatory CD4+ T cells to expand in a lymphopenic environment. However, our data clearly show that IL-2 production by conventional T cells was not critical for the spontaneous proliferation of regulatory T cells in response to lymphopenia, confirming recent data from Setoguchi et al.37 Several studies have recently suggested that IL-15 can replace IL-2 to promote the generation of natural regulatory CD4+ T cells in the thymus.41,42 Thus, we cannot completely rule out the possibility that IL-15 can compensate for IL-2 in our system. However, as IL-15 is not produced by T cells, conventional T cells would have to act both directly by producing IL-2 and indirectly by stimulating IL-15 production by epithelial cells, phagocytes, or myoblasts for this hypothesis to hold true.
When help was provided, the frequency of regulatory CD4+ T cells undergoing strong expansion in response to lymphopenia was similar to that for conventional CD4+ T cells in both C57BL/6 and NOD mice (Figures 2B and 5A). Nevertheless, whereas CD25+ or CD25− T-cell clones expanded to similar extents in C57BL/6 mice (CD25−, 0.027 × 106 ± 0.008; CD25+, 0.035 × 106 ± 0.018; P = .26; Figure 2B), in NOD mice conventional CD4+ T cells tended to expand more strongly in response to lymphopenia than regulatory CD4+ T cells, at the single-cell level (CD25−, 0.36 × 106 ± 0.16; CD25+, 0.022 × 106 ± 0.010; P = .009; Figure 5A). On one hand, King et al showed that T cells from NOD mice produce more IL-21 than those from C57BL/6 mice, subsequently displaying an up-regulation of the surface expression of the IL-21 receptor.21 Moreover, IL-21 has been shown to enhance conventional T-cell proliferation, without reversing the anergic proliferative phenotype of regulatory T cells.43 Finally, several studies have suggested that IL-21 may play a major role in the development of inflammatory and autoimmune diseases,44 including diabetes.21,45,46 Thus, the greater capacity of conventional than of regulatory CD4+ T cells to expand strongly in NOD mice may reflect differences in the ability of these cells to proliferate in response to excess IL-21.
A difference in the potential of regulatory and conventional CD4+ T-cell clones to expand in response to lymphopenia may lead to an unbalance between the 2 T-cell compartments during immune reconstitution in NOD mice. Accordingly, when small numbers of CD4+ T cells from NOD mice (<5 × 105) were injected into lymphopenic recipient mice, that is, when the degree of initial lymphopenia was severe, the proportion of regulatory CD4+ T cells (Foxp3+ cells) among the CD4+ T cells recovered 28 days later was significantly lower than that for control NOD mice (Figure 5B). This lower proportion of regulatory T cells was correlated with the presence of mononuclear cell infiltrates in the intestine and stomach. By contrast, with similar inputs in C57BL/6 mice, spontaneous proliferation in response to lymphopenia did not lead to lower levels of regulatory CD4+ T cells, and no sign of autoimmunity was observed. This finding contrasts with those of Milner et al,47 showing that C57BL/6 Rag2−/− recipient mice injected with 3 × 104 CD4+ T cells developed severe multiorgan inflammatory disease. Milner et al assessed disease 3 to 4 months after the injection of CD4+ T cells, whereas our analysis was conducted 28 days after transfer. We therefore cannot rule out the possibility that our C57BL/6 recipient mice injected with 5 × 103 or 5 × 104 CD4+ T cells might also have developed diseases similar to those described by Milner et al over longer periods of time.47
Twenty-eight days after the transfer of less than 5 × 105 CD4+ T cells, mononuclear cell infiltrates in the intestine and the stomach were observed only in the NOD background. These NOD recipient mice did not develop diabetes or insulitis. This is not surprising, because the induction of diabetes requires the transfer of both CD4+ and CD8+ T lymphocytes48 and a longer time period. We therefore used BDC2.5 TCR-transgenic NOD mice to study the development of diabetes after the transfer of CD4+ T cells. In this model, we show that the prevalence of diabetes depends on the absolute number of CD4+ T cells from BDC2.5 NOD mice injected and is correlated with the proportion of regulatory T cells among the CD4+ T cells recovered from the pancreatic LNs of injected recipients. Moreover, if the CD4+ T-cell population injected into the mice was depleted of CD25-expressing cells before transfer, the recipient mice developed diabetes regardless of the number of CD4+ T cells initially injected. Thus, after the injection of small numbers of CD4+ T cells, spontaneous proliferation in NOD mice led to an imbalance between the regulatory and conventional CD4+ T-cell compartments, at the expense of regulatory T cells, triggering the development of autoimmune disorders.
In both C57BL/6 and NOD mice, the proportion of CD4+ T cells undergoing spontaneous proliferation in response to lymphopenia is quite low. Peripheral immune reconstitution by strong expansion of a minority of T cells might alter the repertoire, resulting in the overrepresentation of T cells with the highest affinity for self. We show in this study that, in C57BL/6 mice, the resulting repertoire includes a proportion of regulatory T cells at least as large as that observed in a normal mouse and possibly even larger, in cases of mild lymphopenia. Moreover, no autoimmune disorder was observed in lymphopenic C57BL/6 mice reconstituted with small or large numbers of CD4+ T cells in the 28 days after the transfer. By contrast, differences in the ability of conventional and regulatory T cells to expand in response to lymphopenia in NOD mice lead to changes in the quality of the peripheral T-cell pool, with the overrepresentation of conventional T cells, resulting in the onset of autoimmune diseases in the context of severe initial lymphopenia. Thus, lymphopenia alone is not sufficient to induce autoimmunity (C57BL/6 mice), but, when coupled to another genetic susceptibility (NOD mice), lymphopenia may act as a signal triggering the development of autoimmune disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Fondation pour la Recherche Médicale and by a grant for young investigators from the French National Research Agency. B.M. was supported by a PhD fellowship from the Fondation pour la Recherche Médicale.
Authorship
Contribution: A.L.C. performed research, analyzed data, and designed the research; M.-C.G., C.A., C.B., M.P.-R., E.L., B.B., and B.M. performed research and analyzed data; F.L. designed the research; and B.L. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno Lucas, Department of Immunology, Cochin Institute, Inserm Unité 567, CNRS UMR 8104, René Descartes University, Cochin Hospital, 75014 Paris, France; e-mail: bruno.lucas@inserm.fr.