Abstract
The germinal center (GC) is a transient lymphoid tissue microenvironment that fosters T cell–dependent humoral immunity. Within the GC, the B cell–specific enzyme, activation-induced cytidine deaminase (AID), mutates the immunoglobulin locus, thereby altering binding affinity for antigen. In the absence of AID, larger GC structures are observed in both humans and mice, but the reason for this phenomenon is unclear. Because significant apoptosis occurs within the GC niche to cull cells that have acquired nonproductive mutations, we have examined whether a defect in apoptosis could account for the larger GC structures in the absence of AID. In this report, we reveal significantly reduced death of B cells in AID−/− mice as well as in B cells derived from AID−/− bone marrow in mixed bone marrow chimeric mice. Furthermore, AID-expressing B cells show decreased proliferation and survival compared with AID−/− B cells, indicating an AID-mediated effect on cellular viability. The GC is an etiologic site for B-cell autoimmunity and lymphomagenesis, both of which have been linked to aberrant AID activity. We report a link between AID-induced DNA damage and B-cell apoptosis that has implications for the development of B-cell disorders.
Introduction
A fundamental hallmark of humoral immunity is the ability to produce class-switched antibodies that have enhanced affinity for antigen. This process, termed affinity maturation, allows clonally selected B cells to refine their response to theoretically target any antigen with high specificity. After activation, B cells mutate the immunoglobulin (Ig) locus in a process known as somatic hypermutation (SHM).1 Mutated clones that have acquired increased affinity for antigen preferentially expand over their low-affinity counterparts. The selection process occurs in the germinal center (GC), a transient microenvironment that forms in secondary lymphoid tissue shortly after antigen exposure.2 The GC provides a competitive setting for B cells whereby ineffectual clones are actively cleared from the system via apoptosis, although the processes that govern this selection still remain vague.
SHM and class switch recombination (CSR) are initiated by the enzyme activation-induced cytidine deaminase (AID),3,4 which deaminates cytidines to uridines specifically at the Ig locus.5-10 AID-generated uridines are then engaged by various DNA repair pathways that either lead to the generation of point mutations in the antibody variable region or recombinogenic events that lead to CSR. AID−/− mice lack mutations at their Ig locus and are incapable of producing class-switched antibodies.4 Interestingly, these mice were previously shown to harbor an abnormally high proportion of splenic GC B cells.11 This phenotype is also recapitulated in humans with AID deficiencies.3 Although a link between reduced gut immunity (resulting from an inability to produce mucosal IgA) and peripheral GC formation was hypothesized to account for these abnormalities, this remains to be conclusively proven, and the possibility of a B cell–intrinsic effect that could explain the profound expansion of GC B cells has not been examined.
GC B cells represent a unique lymphoid compartment of actively proliferating cells where numerous apoptotic factors must synergize to induce the elimination of nonproductive clones. Factors contributing to the intrinsic pathway of apoptosis, such as Bcl-2, Bcl-xL, and Bim,12-17 and the extrinsic pathway, such as Fas,18-22 have been implicated in GC selection. The unique physiology of these cells makes them highly susceptible to disease progression, often serving as etiologic sites for autoimmune and malignant B cells.13,23-26 Recent evidence has implicated AID as a fundamental contributor to the genetic aberrations that lead to these disease phenotypes.24,27-29 Given the importance of apoptosis as a parameter for both GC B-cell selection and lymphomagenesis, we investigated the relationship between AID-induced DNA mutation and cell death to understand how this enzyme may impinge on survival and death within the GC niche. Here we report that many of the potentially harmful AID-induced genetic alterations may indeed lead to the death of these cells within the GC.
Methods
Mice and immunizations
Wild-type (WT) and AID−/− mice bred on the C57BL/6 background (obtained from Charles River Laboratories and Tasuku Honjo, Kyoto University, Kyoto, Japan, respectively) used for antibiotic and apoptotic experiments were 10 to 12 weeks of age before immunizations. Mice were immunized once intraperitoneally with 200 μg of the hapten 4-hydroxy-3-nitrophenyl (NP) conjugated to chicken gamma globulin (CGG; Biosearch Technologies) and precipitated in a 1:1 ratio with alum (Pierce Biotechnology). At 4 weeks or 8 weeks of age, mice were administered oral antibiotics via drinking water. The following antibiotics and concentrations were used for a period of 1 or 2 months: ampicillin 1 g/L (BioShop Canada Inc), metronidazole 1 g/L (Sigma-Aldrich), vancomycin 0.5 g/L (Sigma-Aldrich), and neomycin 1 g/L (BioShop Canada Inc). Antibiotics were administered daily to the drinking water, at the indicated concentrations.
B6.SJL-Ptprca mice (C57BL/6 mice congenic for the SJL CD45.1 allele) were purchased from The Jackson Laboratory. B6.SJL-Ptprca mice were crossed with AID−/− mice to produce an F1 generation positive for both the .1 and .2 alleles of CD45. These F1 mice were used as recipients for B6.SJL-Ptprca and AID−/− bone marrow (BM); F1 mice were irradiated twice with 550 cGy using a Gammacell 40 Exactor (MDS Nordion). BM cells were isolated from both AID−/− and B6.SJL-Ptprca (WT) mice, suspended in phosphate-buffered saline (PBS) at 2 × 107 cells/mL, and mixed at a ratio of 3:1, 1:1, or 1:3. Irradiated mice were reconstituted with 2 × 106 cells by injecting 100 μL of the BM cell suspension into the lateral tail vein. Control BM chimeric mice were produced in the same manner as mixed BM chimeric mice but were reconstituted with a mixture of C57BL/6 and B6.SJL-Ptprca BM cells. BM chimeric mice were kept on antibiotic water containing 2 g/L of neomycin for 1 week after irradiation. After 6 to 8 weeks, BM chimeric mice were immunized intraperitoneally with 100 μg NP-CGG precipitated 1:1 in alum. All animal study protocols were approved by the University of Toronto's Division of Comparative Medicine Committee.
Flow cytometric analyses
Spleens of nonchimeric mice were harvested 11 days after initial immunization. Splenocytes were obtained by mashing tissue through a 70-μm cell strainer and lysing red blood cells in 1 mL ammonium chloride/potassium bicarbonate lysis buffer for 1 minute at room temperature. Cells were then blocked in PBS with 2% fetal calf serum (HyClone) and anti-FcγIII/II antibody (clone 2.4G2). To visualize the GC by flow cytometry, combinations of the following antibodies were used: anti–mouse B220 (eBioscience; clone RA3-6B2), anti–mouse IgD (eBioscience; clone 11-26c), peanut agglutinin (Biomeda), anti–mouse Fas (eBioscience; clone 15A7), and anti–mouse GL7 (BD Biosciences; clone GL7).
Spleens of mixed BM chimeric mice were harvested 7, 11, 14, and 21 days after immunization. Splenocytes were isolated as described above and stained with anti–mouse B220 or anti–mouse CD19 (eBioscience; clone ebio1D3), anti–mouse GL7, anti–mouse Fas, anti–mouse CD45.1 (eBioscience; clone A20), and anti–mouse CD45.2 (eBioscience; clone 104). All stained cells were analyzed using a FACSCalibur or FACSAria flow cytometer (BD Biosciences) and FlowJo software (TreeStar Inc) plotting with biexponential x- and y-axes.
Immunofluorescent microscopy and immunohistochemistry
Extracted spleens of mice were flash frozen in Tissue-Tek OCT (QIAGEN) and stored at −80°C. Thawed tissue samples were cryosectioned at a 5-μm thickness and stained for GCs using fluorescently labeled anti–mouse IgD and peanut agglutinin. Alternatively, spleen sections were labeled with fluorescein isothiocyanate (FITC)–GL7 and IgD-biotin followed by anti–FITC-alkaline phosphatase plus streptavidin-conjugated horseradish peroxidase (both Roche) and developed using horseradish peroxidase and alkaline phosphatase–specific kits according to the manufacturer's instructions (Vector Laboratories). These sections were compared with a serial section stained with anti-CD45.1 and anti-CD45.1 to identify GC occupied by AID−/− versus WT B cells. Stained sections were visualized using a Leica confocal fluorescent microscope and analyzed using Volocity software (Improvision) and Photoshop 7.0 (Adobe).
Primary B-cell stimulation
Spleens from 10- to 12-week-old mice were harvested and pure B cells isolated using a magnetic negative selection B-cell enrichment kit (StemCell Technologies) according to the manufacturer's instructions. B-cell purity was assessed by flow cytometry and found to be at least more than 95% B220 positive. Purified cells were seeded at 4 × 105 cells/well of a 96-well plate and exposed to 25 μg/mL lipopolysaccharide (Escherichia coli serotype 055:B5; Sigma-Aldrich) or 10 μg/mL anti–mouse IgM f(ab′)2 (Jackson ImmunoResearch Laboratories; 115-006-020) and anti–mouse CD154 (CD40L; eBioscience; clone MR1). Cultured cells were grown in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal calf serum (HyClone) and 50 μm β-mercaptoethanol (Invitrogen) at 37°C and 5% CO2. To assess proliferation, cultured cells were stained with the dye carboxyfluorescein diacetate succinimidyl ester (Invitrogen) and monitored every 24 hours for dilution of the dye corresponding to cell proliferation. To assess apoptosis, cultured cells were stained with annexin V (eBioscience) at room temperature for 15 minutes and a caspase 3–specific substrate (BioVision) at 37°C for 30 minutes and assessed by flow cytometry.
In vivo apoptosis assays
To assess apoptosis levels in the GC in vivo, harvested splenocytes were first surface stained for GC markers as mentioned in “Flow cytometric analyses.” For caspase activity, cells were treated with the fluorescently conjugated CaspGLOW reagents specific to caspases 3, 8, and 9 (BioVision) according to the manufacturer's instructions with minor alterations. Briefly, 106 cells were surface stained and then treated with CaspGLOW reagent for 30 minutes at 37°C in PBS and assessed immediately via flow cytometry. To assess late-stage apoptosis in vivo, the APO-BRDU kit (BD Biosciences) was used according to the manufacturer's instructions. Briefly, after surface staining, 1 to 2 × 106 cells were fixed in 1% paraformaldehyde. Cells were then incubated with terminal deoxynucleotidyl transferase and 5-bromo-2-deoxyuridine, stained with FITC-labeled antibromodeoxyuridine antibody, and assessed immediately via flow cytometry.
In vitro cell culture
Ramos is an Epstein-Barr virus–negative human Burkitt lymphoma cell line. Cells were grown in Iscove modified Dulbecco medium (Invitrogen) and 10% bovine calf serum (HyClone). Western blots for AID expression were performed as previously described30 with mouse anti–human AID (Cell Signaling Technology) and rabbit polyclonal anti-β actin (Abcam) as per the manufacturer's instructions. A naturally low-expressing AID clone of Ramos (R1) was electroporated with 10 μg linearized pCEP4-AID in Iscove modified Dulbecco medium at 250 V, 960 μF, plated into 96-well plates, and selected for transfectants using 0.8 mg/mL hygromycin B to express human AID in this cell line. Apoptosis was measured in Ramos cells by annexin V staining as in “Primary B-cell stimulation.”
dsDNA break analyses
Double-stranded DNA (dsDNA) breaks were assayed using a plasmid integration assay as previously described30 using electroporation conditions as previously described.8 Transient transfection efficiencies were calculated by electroporation of Ramos cells with the pmaxGFP plasmid (Amaxa) and analyzed by flow cytometry 24 hours later.
Statistical analysis
All analyses were performed using GraphPad Prism. For Student t tests, 2-way analysis of variance, and Mann-Whitney tests, P values of .05 or less were considered significant.
Results
Splenic GCs are larger in the absence of AID
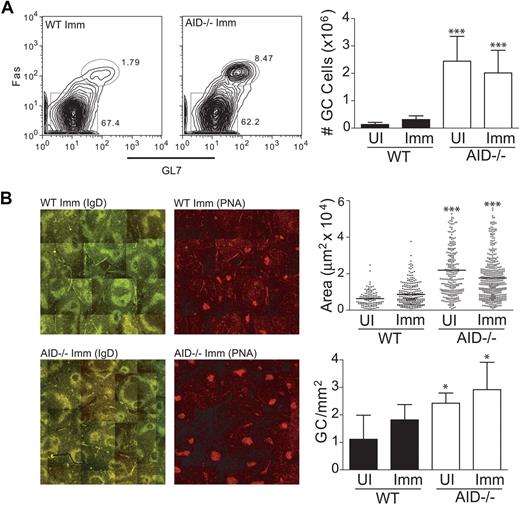
To examine the size and structure of GCs that form in the absence of AID, mice were immunized with the T-dependent antigen NP-CGG. The cellular response was examined 11 days after immunization by staining splenocytes for GC-specific cell surface markers. In WT mice, we observed an increase in the number of GC B cells (B220+, GL7+, Fas+) upon immunization with NP-CGG (Figure 1A). Confirming earlier reports,11 we found that AID−/− mice had larger numbers of GC B cells compared with WT controls both in the presence and absence of immunization (Figure 1A). We repeated these analyses using an alternate GC-specific surface stain (B220+, PNAhigh, IgDlow) and obtained similar results (data not shown).
AID deficiency leads to an abnormal accumulation of GC B cells in the spleen. (A) WT and AID−/− mice were immunized with NP-CGG; and 11 days later, cells were harvested to analyze GC formation via flow cytometry. B220+ GC cells were gated based on expression of Fas and GL7 on the cell surface. Absolute numbers of GC B cells were determined before and after immunization. (B) Splenic tissue was sectioned and stained for immunofluorescent microscopy. GCs were identified as IgDlow and PNAhigh (shown in separate panels) at original magnification ×10 using a Leica confocal fluorescent microscope, and individual images were spliced together to form a complete topography of the spleen. Average GC areas and frequency of formation (identified as PNAhighIgDlow clusters located within IgD+ follicles) were determined and illustrated using Volocity software (Improvision) and Photoshop 7.0 (Adobe). Statistics represent comparisons between WT unimmunized (UI) versus AID−/− UI and WT immunized (Imm) versus AID−/− Imm. Data are mean ± SD of n = 5 to 8 mice per genotype. *P ≤ .05. ***P ≤ .001.
AID deficiency leads to an abnormal accumulation of GC B cells in the spleen. (A) WT and AID−/− mice were immunized with NP-CGG; and 11 days later, cells were harvested to analyze GC formation via flow cytometry. B220+ GC cells were gated based on expression of Fas and GL7 on the cell surface. Absolute numbers of GC B cells were determined before and after immunization. (B) Splenic tissue was sectioned and stained for immunofluorescent microscopy. GCs were identified as IgDlow and PNAhigh (shown in separate panels) at original magnification ×10 using a Leica confocal fluorescent microscope, and individual images were spliced together to form a complete topography of the spleen. Average GC areas and frequency of formation (identified as PNAhighIgDlow clusters located within IgD+ follicles) were determined and illustrated using Volocity software (Improvision) and Photoshop 7.0 (Adobe). Statistics represent comparisons between WT unimmunized (UI) versus AID−/− UI and WT immunized (Imm) versus AID−/− Imm. Data are mean ± SD of n = 5 to 8 mice per genotype. *P ≤ .05. ***P ≤ .001.
The increased number of GC B cells in AID−/− mice might be the result of enlarged GC size and/or more numerous GC seeding in the spleen. Thus, we performed immunofluorescent staining of frozen spleen sections to discriminate between these 2 possibilities. We found the average area of GCs (PNAhighIgDlow clusters located within IgD+ follicles) measured in both unimmunized and immunized AID−/− spleens to be significantly larger than the average area of GCs measured in WT spleens (Figure 1B). Furthermore, GCs in the AID−/− spleen formed at a significantly higher frequency than in the WT spleen. Together, these data indicate that an AID deficiency leads to larger and more numerous GCs both before and after immunization.
Administration of broad-spectrum antibiotics to AID−/− mice does not abrogate GC expansion
It was previously shown that AID deficiency may contribute to a reduced ability to retain intestinal commensal bacteria growth ostensibly because of the inability to produce mucosal IgA.11 Subsequent bacterial escape into the periphery has been hypothesized as a potential explanation for the spontaneous GC formation observed in these mice. Furthermore, short-term treatment of these mice with broad-spectrum antibiotics was able to ablate spontaneous GC formation in the peripheral tissue of 5-week-old mice.11 However, GC B cells are known to accumulate over time in the spleen of AID−/− mice,11 and the long-term effects of antibiotics on this process remain unknown. In 2 independent experiments, we administered broad-spectrum antibiotics to mice over a period of 1 or 2 months to detect their effects on GC B-cell populations after immunization. Consistent with previous reports,11 antibiotic treatment had a dampening effect on the spontaneous GC B-cell population in unimmunized AID−/− mice (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), although GC B cells in these mice were still present in high numbers in the spleen. Importantly, antibiotic-treated AID−/− mice still had increased numbers of GC B cells compared with their WT counterparts, suggesting that this treatment did not affect the abnormal retention of these cells in the AID−/− mouse.
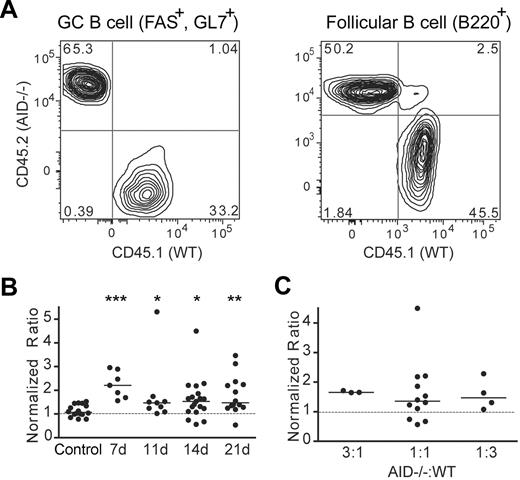
Preferential accumulation of AID−/− GC B cells in mixed BM chimeric mice
To further discount the possibility that GC expansion in AID−/− mice is the result of uncontrolled regulation of intestinal microflora, we examined the GC response of AID−/− B cells in a mixed BM chimeric system. Host mice (CD45.1+, CD45.2+) were irradiated and reconstituted with a mixture of BM cells from CD45.1-congenic WT and CD45.2 AID−/− donor mice. After immunization, we tracked the populations of WT (CD45.1+, CD45.2−) and AID−/− (CD45.1−, CD45.2+) B cells in both the follicle (B220+, GL7−, Fas−) and in the GC (B220+, GL7+, Fas+; Figure 2A). The relative accumulation of AID−/− versus WT GC B cells was calculated by tabulating the ratio of AID−/− B cells to WT B cells in the GC compared with the same ratio in the follicle where B cells are quiescent and selection is not a factor. In control chimeras injected with WT CD45.1 and WT CD45.2 BM, B cells derived from both WT congenic donors were equally represented within the GC niche (the median normalized ratio was ∼ 1), thus validating our approach (Figure 2B; control). In contrast, normalized ratios for all time points after immunization in the AID−/−/WT mixed BM chimeric mice were significantly higher than the control, indicating a preferential survival/accumulation of AID−/− GC B cells compared with WT GC B cells (Figure 2B). Importantly, this skew toward AID−/− GC B cells was evident even when chimeric mice were reconstituted with 3-fold more WT BM versus AID−/− BM cells (Figure 2C). These data are consistent with the observation of an expanded GC compartment in nonchimeric AID−/− mice and conclusively demonstrate that this phenomenon is not entirely dependent on a breach in gut immunity and the loss of IgA.
AID−/− GC B cells outnumber WT GC B cells in mixed BM chimeric mice. (A) Mixed BM chimeric mice were immunized with NP-CGG, and splenocytes were analyzed by flow cytometry 7, 11, 14, and 21 days later. B220+ B cells were divided into follicular B cells (GL7− Fas−) or GC B cells (GL7+ Fas+). The level of chimerism among splenic follicular and GC B cells was determined by taking the ratio of the percentage of AID−/− B cells (CD45.1− CD45.2+) to WT B cells (CD45.1+ CD45.2−). The chimerism among GC B cells was then divided by the chimerism among follicular B cells to produce a normalized ratio whereby values < 1 indicate a skew toward AID−/− B cells. (B) Normalized ratios of splenic GC B cells from mixed BM chimeric mice from various time points after immunization were compared with those of control BM chimeric mice harvested at day 14 after immunization. Each dot represents an individual mouse, solid lines represent median values, and the dotted line represents a normalized ratio of 1. Statistics represent comparisons between experimental mice and the control chimeras. Data are pooled from 6 independent experiments. *P ≤ .05; **P ≤ .01; and ***P ≤ .001. (C) Normalized ratios of splenic GC B cells from mixed BM chimeric mice reconstituted with different ratios of AID−/−: WT BM cells and harvested at day 14 after immunization are compared with one another.
AID−/− GC B cells outnumber WT GC B cells in mixed BM chimeric mice. (A) Mixed BM chimeric mice were immunized with NP-CGG, and splenocytes were analyzed by flow cytometry 7, 11, 14, and 21 days later. B220+ B cells were divided into follicular B cells (GL7− Fas−) or GC B cells (GL7+ Fas+). The level of chimerism among splenic follicular and GC B cells was determined by taking the ratio of the percentage of AID−/− B cells (CD45.1− CD45.2+) to WT B cells (CD45.1+ CD45.2−). The chimerism among GC B cells was then divided by the chimerism among follicular B cells to produce a normalized ratio whereby values < 1 indicate a skew toward AID−/− B cells. (B) Normalized ratios of splenic GC B cells from mixed BM chimeric mice from various time points after immunization were compared with those of control BM chimeric mice harvested at day 14 after immunization. Each dot represents an individual mouse, solid lines represent median values, and the dotted line represents a normalized ratio of 1. Statistics represent comparisons between experimental mice and the control chimeras. Data are pooled from 6 independent experiments. *P ≤ .05; **P ≤ .01; and ***P ≤ .001. (C) Normalized ratios of splenic GC B cells from mixed BM chimeric mice reconstituted with different ratios of AID−/−: WT BM cells and harvested at day 14 after immunization are compared with one another.
GCs seeded by AID−/− B cells are more numerous and larger than those seeded by WT B cells
To better describe the abnormal accumulation of AID−/− GC B cells in mixed BM chimeric mice, we studied GC size via histologic examination of serial spleen sections. We observed 3 different types of GC: those that were occupied by a mixture of WT and AID−/− B cells (∼ 60% of all GCs), those that were occupied predominantly by WT B cells, and those that were occupied predominantly by AID−/− B cells. In Figure 3A, the top panel represents an example of a GC that exclusively contains AID−/− B cells, whereas the bottom panel represents an example of a GC that is occupied predominantly by WT B cells. Evaluating only GCs that are primarily occupied by WT or AID−/− B cells, we found a statistically significant increase in the number of GCs occupied by AID−/− B cells at both 14 and 21 days after immunization (Figure 3B). Furthermore, the average size of AID−/− GCs was significantly greater than WT GCs at day 21. However, because of the skew toward AID−/− GCs, data from day 14 lacked enough WT GCs to obtain statistical significance (Figure 3C). Thus, in a mixed BM chimeric system where WT and AID−/− GC B cells are in direct competition, AID−/− GCs are, on average, larger and more frequent than WT GCs.
GCs occupied by AID−/− B cells are more numerous and larger in immunized mixed BM chimeras. (A) Mixed BM chimeras were generated where irradiated hosts were reconstituted with a 1:1 mixture of WT (CD45.1) and AID−/− (CD45.2) donor BM. Most GCs were occupied by both WT and AID−/− B cells. However, some GCs were occupied by either WT or AID−/− B cells exclusively as evidenced by staining for CD45.1 in the absence of CD45.2 in the GL7+ GC, or vice versa. GCs were enumerated (B) and measured for surface area (C) at day 7 and day 14 after immunization. Median values are shown in panel C. Eight mixed BM chimeric mice were evaluated at each time point. Statistics represent comparisons between WT GC versus AID−/− GC. *P ≤ .05. ***P ≤ .001.
GCs occupied by AID−/− B cells are more numerous and larger in immunized mixed BM chimeras. (A) Mixed BM chimeras were generated where irradiated hosts were reconstituted with a 1:1 mixture of WT (CD45.1) and AID−/− (CD45.2) donor BM. Most GCs were occupied by both WT and AID−/− B cells. However, some GCs were occupied by either WT or AID−/− B cells exclusively as evidenced by staining for CD45.1 in the absence of CD45.2 in the GL7+ GC, or vice versa. GCs were enumerated (B) and measured for surface area (C) at day 7 and day 14 after immunization. Median values are shown in panel C. Eight mixed BM chimeric mice were evaluated at each time point. Statistics represent comparisons between WT GC versus AID−/− GC. *P ≤ .05. ***P ≤ .001.
Reduced apoptosis in AID−/− GC B cells
Given the importance of apoptosis as a selective measure within the GC, we examined cellular markers for both early and late stages of cell death to determine whether defects in these pathways were contributing to GC B-cell accumulation in the AID−/− mouse. We focused on caspase 3, the major apoptotic effector enzyme that acts in conjunction with initiator caspases 8 and 9, which are involved in alternate apoptotic pathways and in some cases cell activation.31 To quantify caspase activity, we used a fluorescently conjugated caspase-specific substrate that can be combined with cell-surface staining to compare apoptotic activity between the GC and follicular (ie, background) populations. Figure 4A shows a representative example of caspase 3 staining in follicular (gray filled histogram) versus GC B cells (empty histogram), and a gating strategy is shown for enumerating caspase 3–positive events. Compared with follicular B cells, we found increased activity of all 3 caspases in WT GC B cells, which is expected because of the intense apoptotic selection occurring in this cellular compartment (Figure 4B). Consistent with the observed accumulation of AID−/− GC B cells, however, caspase activity in AID−/− GC B cells remained at background levels. This suggests that reduced apoptosis may be contributing to the phenotypic differences observed between WT and AID−/− GCs.
AID−/− GC B cells have reduced apoptotic activity in vivo. (A) Mice were immunized with NP-CGG and 11 days later were harvested to assess caspase levels in the GC via flow cytometry. Cells were surface stained to mark GC populations and then incubated with fluorescently labeled substrates specific for caspases 8, 9, or 3. Plots were gated either on the follicular B-cell population (B220+, Fas−, PNAlow) or the GC B-cell population (B220+, Fas+, PNAhigh) and assessed for caspase activity (shown is a representative WT sample). (B) Data were accumulated for immunized mice for caspases 8, 9, and 3. Statistics represent comparisons between WT GC versus AID−/− GC. Data are mean ± SD of n = 6 to 9 mice per genotype. (C) Caspase 3 activity for AID−/− and WT GC B cells is shown for immunized mice reconstituted with a 1:1 mixture of WT and AID−/− BM. Data are mean ± SD of n = 6 chimeric mice. (D) Mice were immunized with NP-CGG and 11 days later were harvested and stained to mark GC populations and fixed for use in a flow cytometry–based TdT-mediated dUTP nick-end labeling assay. Gated cells were assessed for apoptotic activity (shown is a representative WT sample). (E) Accumulated data were expressed as a percentage of positive cells. Statistics represent comparisons between WT GC versus AID−/− GC. Data are mean ± SD of n = 6 to 9 mice per genotype. **P ≤ .01. ***P ≤ .001.
AID−/− GC B cells have reduced apoptotic activity in vivo. (A) Mice were immunized with NP-CGG and 11 days later were harvested to assess caspase levels in the GC via flow cytometry. Cells were surface stained to mark GC populations and then incubated with fluorescently labeled substrates specific for caspases 8, 9, or 3. Plots were gated either on the follicular B-cell population (B220+, Fas−, PNAlow) or the GC B-cell population (B220+, Fas+, PNAhigh) and assessed for caspase activity (shown is a representative WT sample). (B) Data were accumulated for immunized mice for caspases 8, 9, and 3. Statistics represent comparisons between WT GC versus AID−/− GC. Data are mean ± SD of n = 6 to 9 mice per genotype. (C) Caspase 3 activity for AID−/− and WT GC B cells is shown for immunized mice reconstituted with a 1:1 mixture of WT and AID−/− BM. Data are mean ± SD of n = 6 chimeric mice. (D) Mice were immunized with NP-CGG and 11 days later were harvested and stained to mark GC populations and fixed for use in a flow cytometry–based TdT-mediated dUTP nick-end labeling assay. Gated cells were assessed for apoptotic activity (shown is a representative WT sample). (E) Accumulated data were expressed as a percentage of positive cells. Statistics represent comparisons between WT GC versus AID−/− GC. Data are mean ± SD of n = 6 to 9 mice per genotype. **P ≤ .01. ***P ≤ .001.
Because antibiotic treatment revealed an immunization-dependent increase in the GC compartment of AID−/− mice, we tested caspase activity in these mice as well. We found that the reduced apoptotic levels found in AID−/− B cells were not affected by antibiotic treatment (supplemental Figure 1B). We extended this study to our mixed BM chimeric system using host mice reconstituted with a 1:1 ratio of WT to AID−/− BM cells (Figure 4C). Focusing on the effector caspase 3, we again found reduced apoptosis in the AID−/− GC B cells compared with WT cells in the same host.
To confirm that our findings extend to late stages of apoptosis within the GC, we examined DNA fragmentation in these cells using the terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. As before, we used flow cytometry and cell-surface markers to compare bromodeoxyuridine incorporation between GC B cells and the background follicular B-cell population (Figure 4D). Substantiating our work with caspases, we found a significant increase in apoptotic cells in WT GC B cells compared with follicular B cells (Figure 4E). Although we also observed an increase in apoptosis in AID−/− GC B cells, the percentage of apoptotic cells from these mice was significantly lower than in WT mice. We repeated these analyses in antibiotic-treated mice and observed the same effect (data not shown). Collectively, we show a diminished rate of apoptosis in AID−/− GC B cells that coincides with the observed accumulation of these cells in the spleen.
AID expression has a direct effect on cell viability
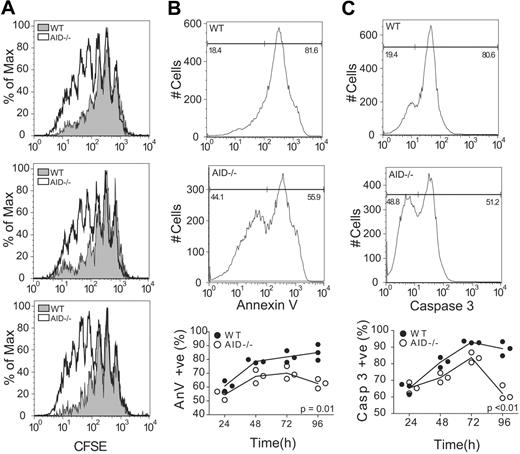
Increased levels of apoptosis observed in WT GC B cells relative to AID−/− B cells may be the result of a cell-intrinsic role for AID by influencing cell survival and/or apoptosis. To test this, we isolated splenic B cells from WT and AID−/− mice and stimulated them by cross-linking the B-cell receptor (BCR; anti-IgM f(ab′)2) and engaging the CD40 receptor (soluble CD40L) to mimic the traditional B-T interaction that precedes GC formation. Stimulated cells were monitored for proliferation (via carboxyfluorescein succinimidyl ester [CFSE] dilution) and apoptosis (via annexin V and caspase 3 staining). B-cell activation led to an increase in proliferation of AID−/− cells compared with WT controls after 96 hours (Figure 5A; P = .02). Furthermore, we observed a concomitant decrease in apoptosis in AID−/− B cells compared with the WT controls (Figure 5B-C).
AID−/− B cells show increased proliferation and decreased apoptosis when stimulated ex vivo. (A) B220+ cells were harvested from the spleens of age-matched WT and AID−/− mice. Cells were incubated with CFSE, and proliferation was monitored daily for 96 hours in response to BCR/CD40 engagement. CFSE dilution plots are shown for the 3 replicates 96 hours after stimulation. Stimulated cells were also monitored for apoptosis through the measurement of annexin V binding (B) and caspase 3 activation (C). Representative plots are shown for BCR/CD40-stimulated WT and AID−/− B cells at 96 hours after stimulation. Statistics represent comparisons between WT and AID−/− cells over time. Data are mean ± SD of n = 3 mice per genotype.
AID−/− B cells show increased proliferation and decreased apoptosis when stimulated ex vivo. (A) B220+ cells were harvested from the spleens of age-matched WT and AID−/− mice. Cells were incubated with CFSE, and proliferation was monitored daily for 96 hours in response to BCR/CD40 engagement. CFSE dilution plots are shown for the 3 replicates 96 hours after stimulation. Stimulated cells were also monitored for apoptosis through the measurement of annexin V binding (B) and caspase 3 activation (C). Representative plots are shown for BCR/CD40-stimulated WT and AID−/− B cells at 96 hours after stimulation. Statistics represent comparisons between WT and AID−/− cells over time. Data are mean ± SD of n = 3 mice per genotype.
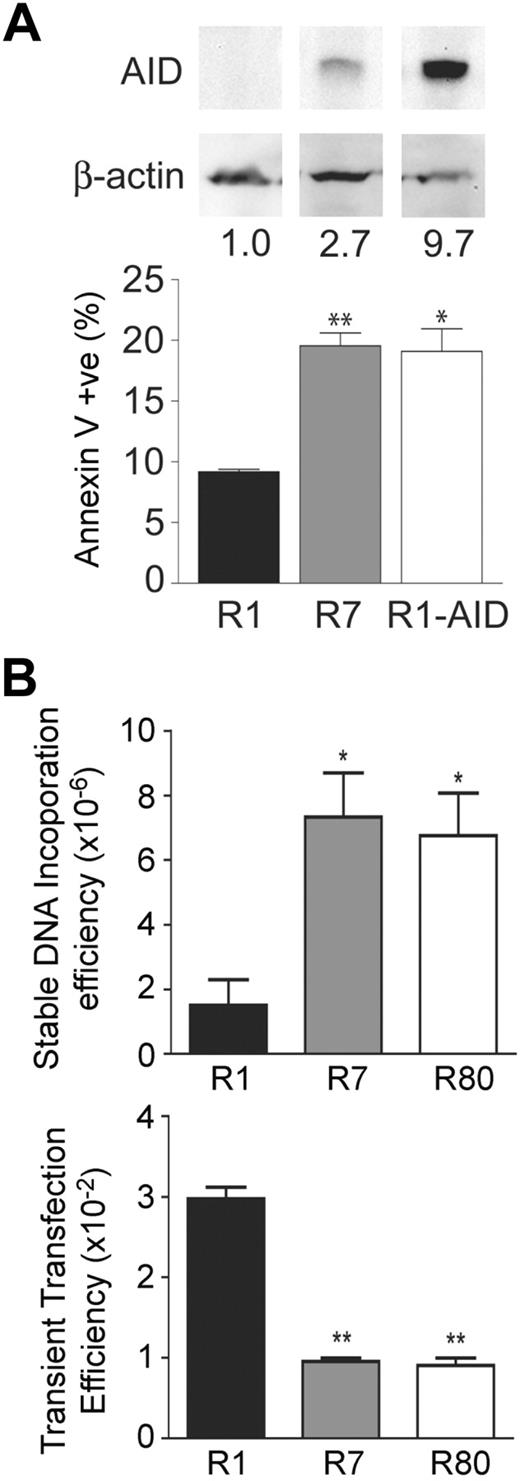
AID levels correlate with apoptosis and dsDNA-break formation in Ramos cells
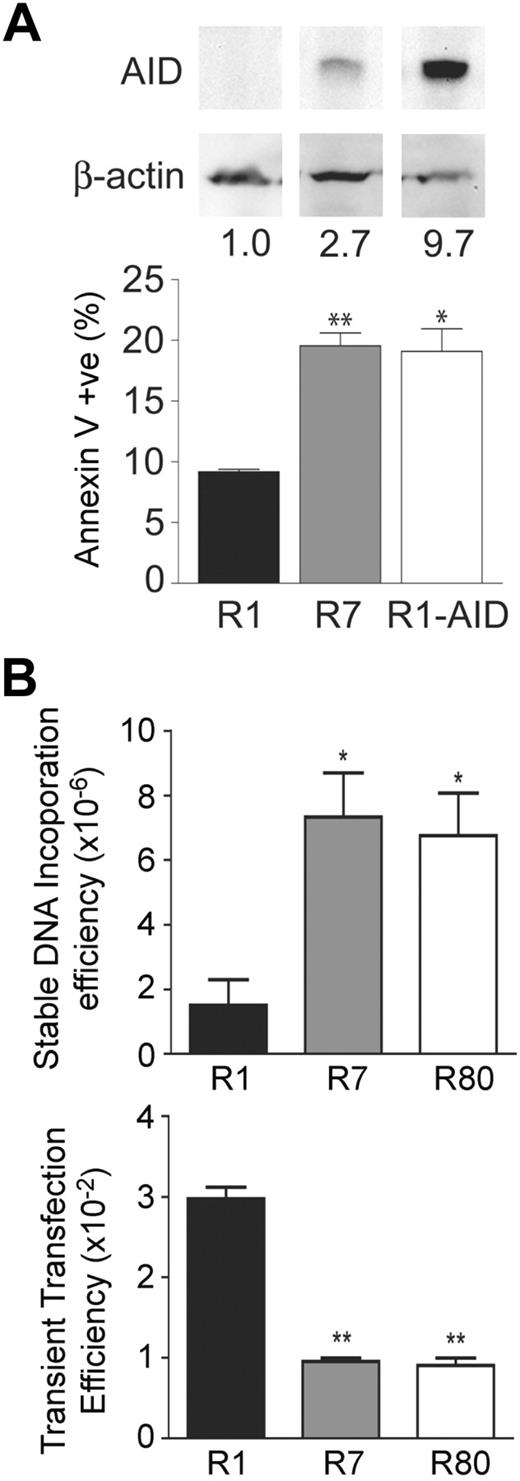
We further explored the association between AID expression and cell death using the GC-derived Burkitt lymphoma cell line Ramos. We found that apoptotic levels, as measured by annexin V binding, were elevated in the Ramos clone R7 that expresses AID (Figure 6A) compared with the AID-negative Ramos clone R1. Furthermore, transgenic expression of AID in the Ramos R1 clone increased the level of apoptosis to the levels seen in the Ramos R7 clone, despite the fact that expression level of AID was approximately 3-fold higher in the Ramos clone R1-AID than the Ramos clone R7 (Figure 6A). This is probably the result of the fact that the mutation rates in both clones are similar,8 which suggests the presence of a factor that is required for AID activity but is present in a limiting amount, as previously discussed.32 These results indicate that the observed changes in apoptotic levels in Ramos cells are an AID-dependent effect.
AID has an intrinsic effect on cell viability. (A) Independently isolated variants of the GC-derived cell lines were assessed for AID levels by Western blotting. A naturally low expressing clone (R1) and high expressing clone (R7) for AID were chosen to look at steady-state apoptotic levels as measured by annexin V binding. R1 cells transgenically expressing AID were also assessed for apoptosis. Statistics represent comparisons between R7 versus R1 and R1-AID and R1. Data are mean ± SD of n = 2 independently cultured clones for each cell line. *P ≤ .05. **P ≤ .01. (B) The AID-negative Ramos 1 clone and 2 AID-high Ramos 7 and 80 clones were assessed for dsDNA break formation by measuring the frequency of stable integration of the pSV2neo E- resistance gene (top panel). These cells were also assessed for DNA uptake by transient transfection with a GFP-expressing plasmid. Statistics represent comparisons between R7 versus R1 and R80 versus R1. Data are mean ± SD of n = 2 or 3 independently cultured clones for each cell line. *P ≤ .05. **P ≤ .01.
AID has an intrinsic effect on cell viability. (A) Independently isolated variants of the GC-derived cell lines were assessed for AID levels by Western blotting. A naturally low expressing clone (R1) and high expressing clone (R7) for AID were chosen to look at steady-state apoptotic levels as measured by annexin V binding. R1 cells transgenically expressing AID were also assessed for apoptosis. Statistics represent comparisons between R7 versus R1 and R1-AID and R1. Data are mean ± SD of n = 2 independently cultured clones for each cell line. *P ≤ .05. **P ≤ .01. (B) The AID-negative Ramos 1 clone and 2 AID-high Ramos 7 and 80 clones were assessed for dsDNA break formation by measuring the frequency of stable integration of the pSV2neo E- resistance gene (top panel). These cells were also assessed for DNA uptake by transient transfection with a GFP-expressing plasmid. Statistics represent comparisons between R7 versus R1 and R80 versus R1. Data are mean ± SD of n = 2 or 3 independently cultured clones for each cell line. *P ≤ .05. **P ≤ .01.
Because of the ability of AID to generate dsDNA breaks, we hypothesized that these events may be responsible for inducing cell-stress pathways. We previously showed that exogenous plasmid DNA incorporates into sites of dsDNA breaks caused by AID in hybridomas.30 Using this same assay, we found approximately 7-fold higher levels of incorporation of the neomycin resistance plasmid in AID-high Ramos 7 and 80 clones33 compared with the AID-negative Ramos 1 clone (Figure 6C), suggesting that the AID-high clones have more dsDNA breaks in their genome. Inaddition, the uptake of DNA by the AID-negative Ramos 1 clone was approximately 3-fold higher than the AID-high Ramos 7 and 80 cells using a transient transfection assay (Figure 6C), showing that the lower level of incorporation in the AID-negative Ramos 1 clone was not the result of the inherent inability of these cells to take up DNA. These data demonstrate that AID expression leads to an approximately 21-fold increase in dsDNA breaks in Ramos cells (ie, 7-fold higher stable transfection efficiency × 3 to correct for reduced transient transfection efficiency), which is consistent with the associated apoptotic phenotype of GC B cells that express AID.
Discussion
Programmed cell death within the GC plays a critical role in the selection of B-cell clones that have modified their Ig locus through SHM.34,35 The importance of apoptosis as it pertains to affinity maturation has been shown previously through the genetic manipulation of apoptotic genes. Transgenic expression of prosurvival factors of the Bcl-2 family, such as Bcl-2 and Bcl-xL,14,15,36 leads to a variety of abnormalities in GC formation and function, such as a reduction in apoptosis within the GC and a subsequent defect in affinity maturation. Studies using mice with either deleted or defective Fas receptors have implicated a similar role for this death receptor in clonal selection.21,22 Furthermore, studies in Bim−/− and Fas-defective mice have reported an abnormal accumulation of antigen-specific GC B cells in the spleen.
AID-induced DNA damage in GC B cells, such as that which occurs during CSR, activates DNA damage response pathways as has been previously shown.37 In contrast, AID−/− GC B cells would undergo expansion within the GC without the associated DNA-damaging events. Our study provides several lines of evidence that a B cell–intrinsic defect in apoptosis is contributing to GC size in the absence of AID. Specifically, we found that BCR/CD40-stimulated AID−/− B cells (1) have a higher proliferative capacity and (2) undergo reduced apoptosis compared with WT B cells (Figure 5). This view was further supported by experiments using Ramos cells (Figure 6) where we conclusively showed a correlation between AID levels and induction of apoptosis. AID-induced strand breaks are crucial to the process of CSR; however, when unresolved, they ultimately lead to cell-cycle arrest and death. Indeed, we observed that AID-induced dsDNA break levels correlated with apoptosis levels in GC B cells and propose that a side effect of the mutagenic activity of AID is the generation of apoptosis-prone B cells that must compete for survival signals within the GC. This is illustrated well in mixed BM chimeric mice where AID−/− B cells are subjected to the normal competitive constraints of the GC milieu and still show evidence of unrestrained accumulation. Taken together, our study is the first to demonstrate an intrinsic apoptosis defect in AID−/− GC B cells that explains their abnormal accumulation in the GC of mice and in humans.
Upon ex vivo stimulation of primary B cells through the BCR and CD40, we observed a proliferative and apoptotic defect stemming from AID expression. We could not, however, recapitulate this result through lipopolysaccharide stimulation of primary cells via the Toll-like receptor 4 (TLR-4; data not shown), confirming earlier reports.4 This finding adds to the growing evidence for unique signaling pathways regulating AID expression through the BCR and other surface receptors.38,39 These studies have shown that signaling through the BCR delays AID expression and down-regulates certain AID-induced processes, such as class-switching, while having no effect on total transcript and protein levels. Furthermore, costimulation of both the BCR and TLR-4 was enough to dampen CSR in a phosphatidylinositol 3-kinase–dependent manner.38 Phosphatidylinositol 3-kinase–signaling interferes with AID activity only when signaled through the BCR, therefore implying a distinct signaling cascade compared with TLR-4. Unlike the BCR and CD40, however, TLR-4 signaling is not known to induce GC formation in vivo; thus, the ex vivo defect reported here might explain why AID deficiency has a GC-specific phenotype regarding apoptosis.40
AID−/− mice do not secrete IgG, which is important to consider as circulating IgG may influence GC B-cell survival via its interaction with the B-cell inhibitory Fc receptor γRIIB1, thus inhibiting antigen receptor signaling.41 However, the absence of circulating IgG cannot explain the larger GCs and reduced GC B-cell apoptosis in AID−/− mice because we also observed enhanced GC B-cell survival as well as increased GC numbers/size in mixed BM chimeric mice (Figures 2–3). Thus, data from the mixed BM chimeric mice demonstrate that a lack of signaling through FcγRIIB1 is not responsible for the abnormal expansion of AID−/− GCs. Furthermore, the observation that there are significantly increased numbers of GCs populated by AID−/− B cells in the mixed BM chimeric mice suggests that a large fraction of GCs occupied by WT B cells are aborted by day 14 of the immune response, whereas GC occupied by AID−/− B cells are persisting. A caveat to this interpretation is that AID−/− B cells may seed more GCs at the onset of the immune response. However, given that we do not see preferential representation of AID−/− pre-GC B cells (naive follicular B cells) in our mixed BM chimeric mice, this scenario is doubtful. In addition, IgA deficiency (and consequently poor control of gut commensal microbes) is not sufficient to explain the enlarged GC compartment observed in the AID−/− mouse because, in both BM chimeric mice as well as antibiotic-treated mice (supplemental Figure 1), AID−/− GC B cells accumulate to a greater degree than WT GC B cells. These data are also corroborated by previous studies in which AID−/− mice were shown to produce tertiary lymphoid organs even when raised in a germ-free facility.42
The GC is often linked to the development of autoimmune B cells, a process associated with abnormal persistence of B cells within this structure. Spontaneous GC formation, such as that found in AID−/− GCs, has also been linked to progression of humoral autoimmunity.43 In humans, a significant portion of patients with inactivating mutations in AID develop autoimmune syndromes by as yet unknown mechanisms.44 Furthermore, aged AID−/− mice were recently reported to develop a fatal autoimmune gastritis linked to activation of peripheral B cells and the development of tertiary lymphoid organs.42 We suggest that the development of autoimmune diseases in AID−/− mice may be linked to improper maintenance of the GC and potential escape of hazardous B-cell clones. These data emphasize the necessity for a fine-tuned balance of AID expression under physiologic conditions that is required for protection against B-cell disease.
In conclusion, we have shown that GC B cells from AID−/− mice have reduced levels of apoptosis contributing to the expansion of these lymphoid structures. Our findings provide clues to the mechanism of B cell–mediated disease development, including humoral autoimmunity and lymphoma. We emphasize the usefulness of the AID−/− mouse as a tool for the study of apoptotic factors that play a role in clonal selection within the GC.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs M. Ratcliffe and M. D. Scharff for comments on this manuscript and the Martin laboratory for helpful discussions.
This work was supported by the Canadian Institutes of Health Research (Ottawa, ON; grant 183815; J.L.G., A.M.). J.L.G. was supported by a Canadian Institutes of Health Research New Investigator Award. A.M. was supported by a Canada Research Chair award.
Authorship
Contribution: A.Z. planned and performed the experiments and wrote the manuscript; B.B. and J.-Y.P. planned and performed crucial experiments; S.R. helped with experiments and contributed essential input into experimental design; and J.L.G. and A.M. conceived the experimental approach and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Martin, Department of Immunology, University of Toronto, Medical Sciences Bldg 5265, Toronto, ON M5S 1A8; e-mail: alberto.martin@utoronto.ca; or Jennifer L. Gommerman, Department of Immunology, University of Toronto, Medical Sciences Bldg 5259, Toronto, ON M5S 1A8; e-mail: jen.gommerman@utoronto.ca.
References
Author notes
*A.Z. and B.B. contributed equally to this work.
†J.L.G. and A.M. contributed equally to this work.