Abstract
Abstract 613
Lenalidomide (Revlimid®) is an oral immunomodulatory agent with clinical efficacy in patients with multiple myeloma (MM). In patients with relapsed/refractory MM, lenalidomide plus dexamethasone improved time to progression (TTP) and overall survival (OS) in comparison with dexamethasone alone. In newly diagnosed MM patients, the current study compares the efficacy and safety of melphalan, prednisone and lenalidomide (MPR) with that of MP alone.
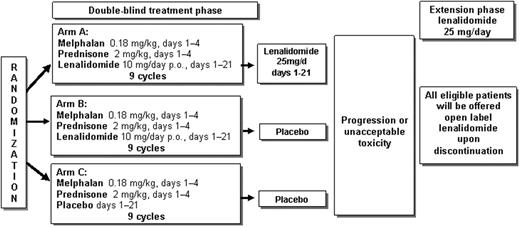
Key inclusion criteria were: ≥65 years of age, newly diagnosed and symptomatic MM. 459 patients were randomly assigned to receive MPR followed by lenalidomide maintenance therapy or MPR followed by placebo maintenance therapy or MP followed by placebo maintenance therapy (Figure 1). The study induction and maintenance phases were followed by an open label lenalidomide extension and a follow-up phase. All patients received aspirin 100 mg/day as thrombo-prophylaxis. The primary endpoint of the study is progression free survival (PFS). The secondary endpoints are OS, time-to-progression, response rate, time to response, response duration, time-to-next anti-myeloma therapy, safety, quality of life and exploratory assessment of cytogenetic abnormalities. Primary comparison is based on the intent-to-treat population comparing PFS between MPR followed by lenalidomide with MP followed by placebo; secondary comparisons are between MPR followed by lenalidomide and MPR followed by placebo, and between MPR followed by placebo and MP followed by placebo.
The first patient was enrolled in February 2007. A pre-planned interim analysis to evaluate the efficacy and safety was performed at 50% information. An independent central adjudication committee determined the assessment and timing of progressive disease prior to the interim analysis. At the interim analysis, it was determined by the Data Monitoring Committee (DMC) that the study had crossed the O'Brien Fleming superiority boundary for the primary endpoint, demonstrating a highly statistically significant improvement in PFS for patients treated with MPR compared with MP as first-line treatment for MM patients. The topline results will be availabel at the time of the meeting.
MPR is an effective and safe regimen for front-line use in MM. PFS was significantly improved in patients who received MPR followed by lenalidomide maintenance compared with those who received MP followed by placebo maintenance. MPR followed by lenalidomide maintenance is a new therapeutic option and can be considered a new standard for patients older than 65 years old.
Palumbo:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmion: Honoraria, Membership on an entity's Board of Directors or advisory committees. Off Label Use: Lenalidomide is not approved for first line use in multiple myeloma. Dimopoulos:Celgene: Honoraria. Delforge:Janssen-Cilag: Consultancy, Honoraria; Celgene: Honoraria, Speakers Bureau. Kropff:Ortho Biotech: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau. Foa:Celgene: Membership on an entity's Board of Directors or advisory committees. Yu:Celgene: Employment. Herbein:Celgene: Employment. Mei:Celgene: Employment. Jacques:Celgene: Employment. Catalano:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.