Abstract
Abstract 4063
Poster Board III-998
In a large, 1-yr Phase 3 clinical trial, patients (pts) with β-thalassemia (aged ≥2 yrs) were randomized to receive deferasirox (Exjade®) or deferoxamine (DFO), with doses assigned according to baseline liver iron concentration (LIC). Pts completing the 1-yr core were permitted to enter a 4-yr extension; those receiving deferasirox continued on this therapy (deferasirox cohort), while those receiving DFO crossed over to deferasirox (crossover cohort). This analysis evaluates the efficacy and safety of deferasirox over 5 yrs.
Based on analyses showing that iron burden and transfusional iron intake need to be considered for appropriate dosing of deferasirox, dose adjustments were permitted in the extension to ensure optimal dosing. Deferasirox dose in the extension was initially based on dose response in the core (deferasirox cohort only) and end-of-core LIC (biopsy or SQUID); subsequent adjustments in steps of 5–10 mg/kg/day were based on serum ferritin (SF) levels and safety markers. Efficacy was assessed by monthly SF levels and LIC at baseline, end of 1-year core and end of study (EOS) (or upon discontinuation). Safety was assessed by incidence and type of adverse events (AEs) and changes in laboratory parameters.
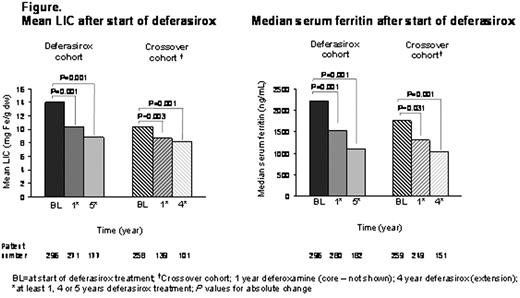
296 pts (deferasirox cohort) and 259 pts (crossover cohort) received ≥1 dose of deferasirox; 181 (61%) & 190 (73%) pts from each cohort respectively completed the extension. Most common reasons for discontinuation: consent withdrawal (n=62) and AEs (n=43). Most common AEs leading to discontinuation: increased ALT [n=5], increased transaminases [n=4], glycosuria [n=4]. 2 deaths occurred during the extension in the deferasirox cohort (cardiac failure, cardiomyopathy); 2 in the crossover cohort (cardio-respiratory arrest, road traffic accident); none considered to be related to study drug. Median duration of deferasirox treatment was 61.2 & 48.1 mths in deferasirox & crossover cohorts, respectively. At start of deferasirox, mean LIC was 14.0 ± 9.8 & 10.4 ± 7.6 mg Fe/g dry weight (dw) and median SF was 2211 & 1758 ng/mL in deferasirox and crossover cohorts, respectively. Transfusion requirements at start of deferasirox were comparable; most pts (81% & 83%, respectively) receiving 7–14 mL/kg/mth. Mean deferasirox dose during study: 21.6 ± 6.4 & 23.2 ± 5.9 mg/kg/d (final actual dose: 24.4 ± 8.7 & 27.0 ± 8.0 mg/kg/d) in deferasirox and crossover groups, respectively. Most pts were receiving 15–<35 mg/kg/day at EOS (75% & 78%, respectively); 11% & 17% were receiving ≥35 mg/kg/day. In pts who received at least 5 yrs of deferasirox and at least 4 yrs in the crossover group, mean absolute change in LIC were –5.3 ± 10.1 mg Fe/g dw (n=173; P<0.001) & –2.4 ± 7.6 mg Fe/g dw (n=99; P<0.001) and median absolute change in SF were –775 ng/mL (range: –10164–2572; n=182; P<0.001) & –371 ng/mL (range: –4498–2636; n=151; P<0.001), respectively (Figure). Percentage of pts with LIC<7 mg Fe/g dw increased from 35% to 45% & SF≤1000 ng/mL increased from 12% to 33% from the start of deferasirox to EOS (LIC: EOS, last available value; SF: EOS, average of at most 3 available values after start of deferasirox).
Most common drug-related AEs (≥5% overall) after start of deferasirox in deferasirox & crossover cohort, respectively: increased blood creatinine (n=42, 14%; n=20, 8%), nausea (n=28, 10%; n=13, 5%), vomiting (n=18, 6%; n=17, 7%), diarrhea (n=13, 4%; n=15, 6%) & rash (n=17, 6%; n=19, 7%). Frequency of drug-related AEs decreased from year to year. In deferasirox & crossover cohorts, 26 (9%) & 11 (4%) pts had 2 consecutive serum creatinine increases >33% above baseline & upper limit of normal (ULN) & 3 (1%) & 2 (1%) pts had ALT >10 x ULN on 2 consecutive visits, respectively, after start of deferasirox.
Long-term treatment with deferasirox (for up to 5 yrs) significantly decreased iron burden in β-thalassemia pts aged ≥2 yrs with an increasing percentage of pts achieving therapeutic goals of LIC<7 mg Fe/g dw and SF≤1000 ng/mL. Significant improvements in LIC and SF were also observed after switching from DFO. Deferasirox was well tolerated over this long-term treatment, and the frequency of AEs decreased over time.
Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genzyme: Membership on an entity's Board of Directors or advisory committees. Perrotta:Novartis: Consultancy, Research Funding. Aydinok:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Vifor International: Membership on an entity's Board of Directors or advisory committees. Piga:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Apopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Griffel:Novartis Pharmaceuticals: Employment, Equity Ownership. Lagrone:Novartis Pharmaceuticals: Employment. Clark:Novartis Pharma AG: Employment. Kattamis:Novartis: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.