Abstract
Abstract 2306
Poster Board II-283
We previously reported excellent outcomes in adult patients with acute lymphoblastic leukemia (ALL) undergoing allogeneic hematopoietic stem cell transplantation (allo SCT) conditioned with medium-dose VP-16 (VP, 30 mg/kg), cyclophosphamide (CY, 120 mg/kg) and fractionated total body irradiation (TBI, 12 Gy) (VP/CY/TBI). In this study, we retrospectively compared the outcomes of this regimen and the standard regimen of CY/TBI.
Clinical data for patients who received the VP/CY/TBI regimen were collected from six SCT centers in Hokkaido, Japan, and data for patients who received the CY/TBI regimen were collected from the Japan Society for Hematopoietic Cell Transplantation database (the Transplant Registry Unified Management Program) and the Japan Marrow Donor Program database. Data for 35 patients and 515 patients who received VP/CY/TBI and CY/TBI, respectively, and who met all of the following criteria were analyzed: SCT having been performed between 1993 and 2007, first time for SCT, aged 15-59 years, diagnosed as having ALL/lymphoblastic lymphoma or acute biphenotypic leukemia, first or second complete remission (CR) at SCT, bone marrow (BM) or peripheral blood stem cells (PBSC) as stem cell source and HLA- phenotypically matched (A, B and DR loci) related donor (MRD) or unrelated donor (MUD). Patients who met at least one of following criteria were excluded: secondary SCT, Burkitt leukemia/lymphoma, cord blood as stem cell, secondary leukemia or T-cell depletion.
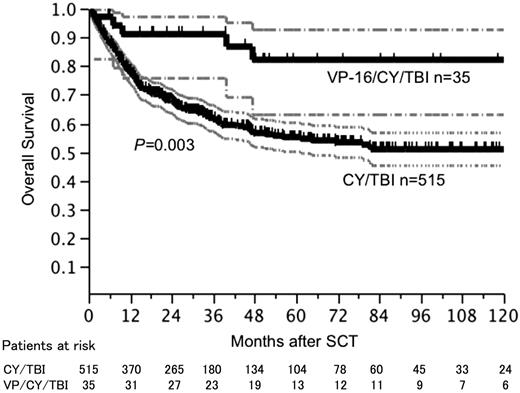
The median age of the patients was 34 years (range: 15-59 years). Two hundred seventy-nine (50.7%) of the patients underwent transplantation from an MRD and 271 (49.3%) of the patients underwent transplantation from an MUD. Four hundred forty-two (80.4%) of the patients received BM and 105 (19.1%) of the patients received PBSC. One hundred fifty-five (28.2%) of the patients were Philadelphia chromosome-positive (Ph+) and 442 (80.4%) of the patients were in CR1 at SCT. Although patients who received VP/CY/TBI were younger (VP/CY/TBI: mean age of 29.6 years; CY/TBI: mean age of 34.1 years, P=0.03), other factors such as Ph chromosome positivity, SCT in CR2, and donor status were not different between the two regimen groups. Five hundred forty-two (98.5%) of the patients achieved neutrophil engraftment at median day of 16. Acute graft-versus-host disease (AGVHD) and grade II-IV AGVHD occurred in 336 (63.5%) and 277 (52.4%) evaluable patients, respectively, at median onset day of 21. Chronic GVHD (CGVHD) occurred in 215 (45.4%) evaluable patients at the median onset day of 120. Incidences of engraftment, AGVHD and CGVHD were not different between the two regimen groups. After a median follow-up period of 37.7 months, 2-year overall survival (OS) and 5-year OS rates were 91.0% and 82.2% in patients who received VP/CY/TBI, respectively, and 68.0% and 55.2% in patients who received CY/TBI, respectively. Survival curves reached plateaus at 47 months after SCT in patients who received VP/CY/TBI and at 82 months in patients who received CT/TBI. OS and disease-free survival (DFS) rates were significantly better in patients who received VP/CY/TBI regimen [OS: logrank P=0.003, DFS: logrank P<0.001 (Figure)]. Although the number of patients who received VP/CY/TBI was relatively small and those patients were younger than the patients who received CY/TBI, the number of the patients who received control regimen of CY/TBI was sufficient for comparison of the regimens. Better outcomes of using VP/CY/TBI were confirmed using multivariate analysis with Cox's regression model [hazard ratio: 0.29 (95% confidential interval: 0.10-0.63)]
Although our analysis has limitations due to its retrospective fashion, analysis of data for a large number of patients who were selected by precise eligibility criteria (conditioning regimen, disease status at SCT, donor status) provided reliable information that medium-dose VP/CY/TBI resulted in a superior survival rate and was more curative than the CY/TBI regimen for adult ALL patients.
Overall survival after SCT according to the conditioning regimens. Blocked lines show survival curve and dotted lines show 95% confidential interval.
Overall survival after SCT according to the conditioning regimens. Blocked lines show survival curve and dotted lines show 95% confidential interval.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.