Abstract
The granulocyte-macrophage colony-stimulating factor (GM-CSF)/interleukin (IL)–3/IL-5 receptor family regulates the production and function of myeloid cells. These cytokines signal through receptor complexes that consist of unique ligand-binding α-chains and common signaling β-chains. IL-5 is distinct from IL-3 and GM-CSF in its capacity to induce eosinophil development, however, the molecular mechanisms that generate functional diversity within this receptor family are mostly unknown. Here, we characterized the selective IL-5Rα–binding adapter protein syntenin in IL-5R function. Syntenin and IL-5Rα colocalize at the plasma membrane and in early endosomal compartments. Manipulation of syntenin expression by ectopic expression or knockdown selectively modulated IL-5R but not GM-CSF receptor signaling, and severely affected IL-5–induced eosinophil differentiation from primary human CD34+ hematopoietic progenitor cells. We found syntenin up-regulated during eosinophilopoiesis but down-regulated during neutropoiesis. Syntenin forms complexes with multiple IL-5Rα chains, suggesting that syntenin-enhanced IL-5R output may result from stabilization of an IL-5–induced oligomeric receptor complex. These data demonstrate that cytokine-specific functions can be transduced by unique receptor α-chain–associating adapter proteins.
Introduction
Leukocyte functions are regulated by intracellular mechanisms that constantly integrate environmental signals. Cytokines and their receptors are key molecules in leukocyte communication that regulate hematopoiesis and immune responses.1 Cytokine receptors have been classified on the shared use of common receptor components such as the common β-chain (βc) for the granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR) family.2 Tightly regulated and complex signaling networks have evolved to ensure robust cellular output of cytokine-mediated signaling input. Adapter and scaffold proteins play a decisive role in these networks by regulating the kinetics and specificity of signal transduction pathways.3 These proteins consist of multiple protein interaction domains that lack enzymatic activity, and modulate the composition of protein complexes and thus which protein substrates are favorably targeted. Adapter molecules may allow the induction of receptor-specific signaling pathways, and cellular functions, however until now there are few examples.
IL-3, IL-5, and GM-CSF are important regulators of myeloid cell differentiation and leukocyte function.4 These cytokines bind to unique α-chains, and then recruit a common β-chain (βc) to form high-affinity receptors. Receptor activation induces overlapping signaling pathways that depend on both α and βc which are preassociated with Janus kinases (JAKs) that activate signal transducer and activators of transcription (STAT).4,5 Other shared signaling pathways include the phosphoinositol-3 kinase (PI-3K)/protein kinase B pathway (PKB/c-Akt) that is crucial for cellular survival, and mitogen-activated protein kinase (MAPK) pathways that drive proliferative responses.
IL-5, IL-3, or GM-CSF can induce distinct hematopoietic stem cell–fate decisions, however, mechanisms that regulate cytokine-specific responses are ill-defined at best.1,4,6 Instructive signals generated by the GM-CSFR mediate myeloid commitment and differentiation of various myeloid lineages, whereas IL-3R plays a role in self-renewal and expansion of myeloid progenitors.7-10 IL-5 specifically promotes eosinophil differentiation and survival from common myeloid progenitors, and plays a dominant role in the development of a subtype of B cells termed B1 cells.11-16 Because the intracellular domains of IL-3Rα, GM-CSFRα, and IL-5Rα are unique, these are excellent candidates to mediate specific signaling pathways and cellular functions. We previously identified syntenin as specific interactor of intracellular IL-5Rα.17 Syntenin is a 32-kDa adapter protein that consist of an N-terminal domain (NTD), and 2 adjacent PDZ domains for which multiple ligands have been described.18 Syntenin interacts through its PDZ domains with the C-terminus of IL-5Rα, whereas its NTD can recruit distinct effector proteins such as the transcription factor SOX4.17
Here, we study the functional relationship between IL-5Rα and syntenin in human CD34+ cells. The data presented suggest a model in which the adapter protein syntenin specifically enhances IL-5R signal output and function by binding to multiple IL-5Rα chains supporting the hypothesis that syntenin stabilizes an IL-5–induced active IL-5R complex. Importantly, syntenin expression was found to be important for IL-5–induced commitment to the eosinophil lineage in primary human CD34+ cells. These data provide novel insights in the molecular mechanisms of cytokine-induced hematopoietic cell–fate decisions and immune cell function.
Methods
Antibodies, DNA constructs, and siRNA
All protocols were approved by the ethics committee of the University Medical Center in Utrecht. The following antibodies were used: PE-conjugated anti–IL-5Rα (CDw125) for immune fluorescence and flow cytometry, anti-CD63 and anti-CD107a (all mIgG1), and isotype controls were from BD Biosciences; rabbit anti–mouse IgG-Cy3 conjugated was from Jackson ImmunoResearch Laboratories. Anti-CD71 (mIgG1), anti-myc clone 9E10, goat antiactin, goat anti–hIL-5Rα (Western blot), rabbit antisyntenin, and antihemagglutinin (HA) clone 12CA5 were from Santa Cruz Biotechnology. Rabbit antibodies against phospho-ERK1/2 and phospho-STAT5 were from NEB, rabbit antiphosphorylated PKB substrate and anti–total ERK1/2, anti–total JAK2, and anti–phospho-JAK2 were from Cell Signaling Technology. Mouse antitubulin was from Sigma-Aldrich. DNA constructs used in this study are pEGFP-C3 (Clontech) containing polymerase chain reaction (PCR)–amplified syntenin17 and pLZRS-IRES EGFP15 containing XhoI/NotI HA-tagged human syntenin; HA-tagged syntenin residues 110 to 298 (PDZ1+2) were cloned by PCR. Syntenin-targeting and control shRNA retroviral expression constructs (nos. 1 and 4, respectively) were previously described.19 Cloning constructs for the protein fragment complementation assay were obtained from Dr Michnick (Université de Montréal). N-terminal YFP1 (residues 1-158) and YFP2 (residues 159-239) fusion products were created by ligating human IL-5R intracellular domain residues 365 to 420 using BspEI/XbaI restriction sites; a C-terminal fusion was created by inserting hIL-5Rα 365 to 420 in front of YFP1 using NotI/ClaI sites. Plasmid backbone was pcDNA3 (Invitrogen). HA-tagged syntenin-PDZ2 residues 194 to 298 were BamHI/NotI cloned in pcDNA3. All constructs were DNA sequence verified. Prevalidated syntenin-targeting siRNA was purchased from Ambion. siRNA no. 1 consists of the sequence 5′-CCUGAAAUGUACACUAGCCTT and siRNA no. 2, 5′-GCAUCUAGACUAAAAGCCATT. A scrambled siRNA control was purchased from Dharmacon.
Transfection, retroviral transduction, and selection of cells
TF-1 cells were maintained in RPMI-1640 containing glutamax (Invitrogen), 10% fetal bovine serum (HyClone Laboratories), penicillin and streptomycin (Invitrogen), 0.5mM β-mercaptoethanol (Merck), and 0.1nM human IL-3 (Richter-Helm Biologics) at 37°C and 5% CO2. Cells (10 × 106/250 μL serum-free medium) were electroporated with pEGFP-C3 or EGFP-tagged syntenin (5 μg) in 0.4-cm cuvettes using a Bio-Rad GenePulser II at 250 V/960 μF. Retroviral supernatants and infections were performed as described.15 Polyclonal EGFP-positive cell lines were sorted using a FACSAria (BD Biosciences). Clones were generated by sorting single EGFP-positive cells in 96-well plates. Eosinophils were magnetically separated from granulocyte preparations by negative selection of CD16, CD14, and CD3 (Miltenyi Biotec).
Confocal studies
Dead cells were removed 24-hours after electroporation by Ficoll separation. The next day, cells were adhered to poly-l-lysine–coated (Sigma-Aldrich) microscope glasses and fixed in PBS containing 3% paraformaldehyde (Merck) for 15 minutes at 21°C and subsequently 100% methanol (Merck) for 30 minutes at −20°C. Cells were preincubated with 10% normal rabbit serum (Jackson ImmunoResearch Laboratories) before PE-conjugated mouse anti–IL-5Rα antibody (5 μg/mL) was added, followed by PBS washes and incubation with 2 μg/mL rabbit anti–mouse Cy3 conjugates (all antibody stainings were in PBS with 10% rabbit serum). Slides were then washed extensively, and cells were mounted in mowiol containing 3% DABCO followed by a glass cover as described.20 CD71, CD63, CD107a, and isotype stainings were all performed by a similar protocol. For IL-5 (Peprotech) stimulations, cells were adhered to glass at 37°C and incubated with 10nM IL-5 and 5 μg/mL monoclonal antibody (mAb) anti–IL-5Rα clone A14 for the indicated time points before washes, fixation, and incubation with Cy3-conjugated rabbit anti–mouse IgG. Slides were examined with a 63× PlanApo objective on a Leitz DMIRB fluorescence microscope (Leica) interfaced with a Leica TCS4D confocal laser microscope (Leica).
IL-5Rα internalization
Generation and maintenance of Ba/F3 cells that stably express hIL-5Rα and βc were described previously.21 Cells were transduced with EGFP or HA-tagged syntenin/EGFP retrovirus and surface expression was analyzed by incubating cells at 4°C with PE-conjugated mouse anti–IL-5Rα antibody and subsequent flow cytometry. To measure IL-5Rα internalization, EGFP and syntenin-transduced cells were washed, and resuspended at 0.5 × 106 cells/mL in medium containing 0.2nM IL-5 for the indicated time points. Reactions were stopped by ice-cold phosphate-buffered saline (PBS) and cells were then stained for IL-5Rα surface levels at 4°C. As controls, isotype stainings were performed and IL-5 was added to prechilled cells on ice for 120 minutes followed by IL-5Rα staining to investigate whether IL-5 could block IL-5Rα–antibody interaction.
Proliferation assay
Polyclonal TF-1 cell lines expressing EGFP, HA-syntenin, or HA-PDZ1+2 were washed and incubated with IL-5 (0.2nM) or GM-CSF (2pM) for 6 days at 2.5 × 105 cells/mL. Every 2 days, cells were 2-fold diluted and cytokines were replenished. Viable cell numbers were determined by light microscopy and trypan blue exclusion in triplicate. Selected clones were washed and plated in 96-well plates supplemented with IL-5 and GM-CSF for 3 days at 1.5 × 104 cells/100 μL. Cells were transferred to tubes and viable cells (live gate, propidium iodide negative, EGFP+) were counted using Flowcount fluorespheres (Coulter Immunotech) in triplicate. U0126 (Biomol International) was added to the cultures at 10μM. Groups were statistically compared by Student t test.
Western blots
TF-1 clones ectopically expressing syntenin or PDZ1+2 were washed twice and cytokine-starved overnight at 106 cells/mL in RPMI with 0.5% serum. The next day, cells were stimulated with IL-5 (0.5nM) or GM-CSF (10pM) for indicated time points. Reactions were stopped by washing with ice-cold PBS, and lysis in Laemmli buffer. Protein levels were quantified according to Lowry,22 and proteins were Western blotted. Membranes were blocked with 5% bovine serum albumin, and antibodies were incubated according to the manufacturer's conditions. Membranes were washed, incubated with appropriate secondary antibodies, and developed by enhanced chemiluminescence and exposure to Kodak XB films. For quantification of IL-5–induced phospho-levels, integrated density values were determined that were corrected for background using ImageJ software (National Institutes of Health, http://rsb.info.nih.gov/ij). The Student t test was applied for statistics.
CD34+ progenitor cell differentiation assays
In vitro differentiation of cord blood CD34+ cells to eosinophils and neutrophils has been described elsewhere.15 Briefly, CD34+ cells were magnetically selected from cord blood and cultures were started by addition of stem cell factor (SCF; 50 ng/mL), FLT-3 (50 ng/mL), IL-3 (0.1nM), GM-CSF (0.1nM), and IL-5 (10pM). Retroviral transductions were performed twice during days 1 and 2 of the culture. Cultures are washed and supplemented at day 3, 7, 10, and 14 with IL-3 (0.1nM) and IL-5 (10pM) to induce eosinophil differentiation or with G-CSF (0.1nM) for neutrophil differentiation. Proliferation rates of cells were determined by counting trypan blue–negative cells. EGFP+ cells were sorted beyond 95% purity at day 17, and cytospins were prepared and stained by May-Grünwald-Giemsa. Eosinophil differentiation was quantified blinded, and cells were scored when they at least resembled type II promyelocytes.
Butyric-induced HL-60 cell differentiation
HL-60 cells were differentiated toward eosinophils by a 2-month preculture in medium at pH 7.7 and subsequent incubation with butyric acid (0.5mM) for 5 days as described.23 Northern blots were prepared as described.24 Blots were sequentially overlaid with 32P-labeled PCR products of approximately 400 base pairs specific for IL-5Rα, syntenin, and GAPDH. Blots were exposed to Kodak AR films, and probes were stripped before reprobing.
Coimmunoprecipitations
Hek293 cells (ATCC) were transfected by addition of PEI-DNA complexes. Two days later, 175 cm2 of cells were resuspended in PBS and incubated with 1 mM DSP (Pierce) for 30 minutes at room temperature. Reactions were stopped with 20mM Tris, pH 7.4, for 15 minutes. Cells were washed, lysed in E1A buffer with protease inhibitors, and incubated with anti–HA-beads (Sigma-Aldrich) for 3 hours. Beads were washed, and boiled for 2 minutes in nonreducing sample buffer before Western blot analysis. Nonreduced cell lysates were prepared before DSP addition to analyze the size of individual components.
Protein fragment complementation
Hek293 cells (ATCC) were grown in 6-well plates in DMEM/10% serum and transfected using PEI-DNA complexes. Cells were analyzed by flow cytometry 48 hours after transfection using a FACSCalibur (Becton Dickinson).
Results
IL-5Rα and syntenin colocalize in TF-1 cells
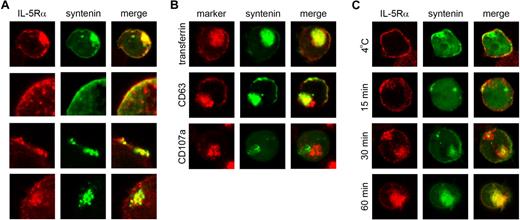
To evaluate syntenin-dependent functions in cells expressing endogenous IL-5Rα, we modulated syntenin expression in the cytokine-dependent, human bone marrow–derived CD34+ TF-1 cell line. These cells are ideally suited to study IL-5Rα–selective signaling because they coexpress IL-3Rα and GM-CSFRα. We analyzed the subcellular distribution of IL-5Rα and syntenin in TF-1 cells, in the presence or absence of IL-5. IL-5Rα and ectopically expressed EGFP-tagged syntenin were found to colocalize both at the plasma membrane and an intracellular compartment (Figure 1A; colocalization is indicated in yellow). In the top panel of Figure 1A, a representative image of an entire cell is shown, the middle panels indicate colocalizing signals at or near the plasma membrane compartment, and the bottom panel indicates colocalization at an intracellular compartment. DsRED and HA-tagged syntenin both showed a similar distribution, whereas the EGFP-tag itself localized throughout the cell or within the nucleus (data not shown). To identify the intracellular compartment to which syntenin localizes, EGFP-syntenin–expressing cells were stained for transferrin receptor (CD71) to visualize early and recycling endosomes, CD63 to stain early and late endosomes, and CD107a to identify lysosomes (Figure 1B). Extensive colocalization at intracellular sites was observed for transferrin receptor, and to some extent for CD63. CD107a did not colocalize with syntenin, suggesting that intracellular syntenin in these cells is localized mainly at the plasma membrane and an early endosomal compartment.
Colocalization of IL-5Rα and syntenin in TF-1 cells. (A) Subcellular distribution of IL-5Rα (indicated in red) and EGFP-tagged syntenin (green) in TF-1 cells maintained in IL-3. Colocalization of IL-5Rα and syntenin is indicated in yellow in the right column (merge). A representative cell from 3 independent experiments is shown in the top panel. The bottom 3 panels show colocalizing structures at higher magnification. (B) Representative examples of EGFP-syntenin–expressing TF-1 cells that were stained for various endocytic markers in red. (C) TF-1 cells ectopically expressing GFP-syntenin were starved for 4 hours in 0.5% serum and incubated with IL-5 and an IL-5Rα–specific monoclonal mouse IgG for 60 minutes at 4°C (top panel), or for different time points at 37°C (bottom panels). Cells were then fixed, and stained using a Cy3-conjugated mAb recognizing mouse IgG (red). Representative examples are shown (n = 3).
Colocalization of IL-5Rα and syntenin in TF-1 cells. (A) Subcellular distribution of IL-5Rα (indicated in red) and EGFP-tagged syntenin (green) in TF-1 cells maintained in IL-3. Colocalization of IL-5Rα and syntenin is indicated in yellow in the right column (merge). A representative cell from 3 independent experiments is shown in the top panel. The bottom 3 panels show colocalizing structures at higher magnification. (B) Representative examples of EGFP-syntenin–expressing TF-1 cells that were stained for various endocytic markers in red. (C) TF-1 cells ectopically expressing GFP-syntenin were starved for 4 hours in 0.5% serum and incubated with IL-5 and an IL-5Rα–specific monoclonal mouse IgG for 60 minutes at 4°C (top panel), or for different time points at 37°C (bottom panels). Cells were then fixed, and stained using a Cy3-conjugated mAb recognizing mouse IgG (red). Representative examples are shown (n = 3).
IL-5 has been reported to trigger IL-5Rαβ internalization, which is subsequently degraded via proteasomal and lysosomal mechanisms.25,26 We studied IL-5Rα internalization by simultaneously incubating TF-1 cells with IL-5 and an IL-5Rα–specific mAb that does not interfere with ligand binding as demonstrated by identical mAb staining after saturation of cells with IL-5 at 4°C (data not shown). Cells were incubated at 37°C for various time points, fixed, and permeabilized, and plasma membrane (–derived) IL-5Rα–binding mAb was detected by Cy3-conjugated anti–mouse antibody (Figure 1C). An identical procedure was performed at 4°C to prevent IL-5Rα internalization (Figure 1C top row). At early time points, and at 4°C, IL-5Rα colocalized with EGFP-syntenin at the plasma membrane, but at later time points clear colocalization was observed at the syntenin-positive intracellular compartment. No staining was observed after incubation of cells with isotype control antibody (data not shown). These results reveal association of IL-5Rα and syntenin at the plasma membrane and internalization into an early endosomal compartment.
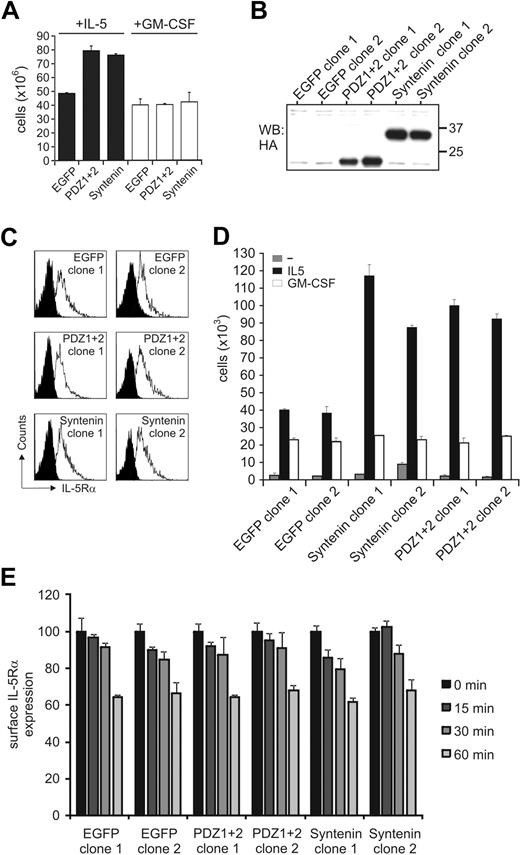
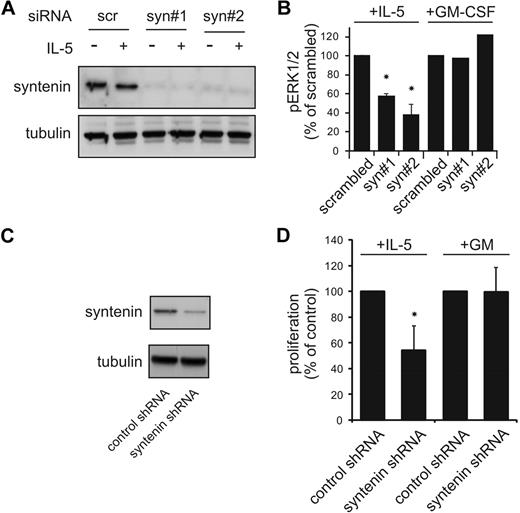
Syntenin expression regulates IL-5–driven proliferation and signaling
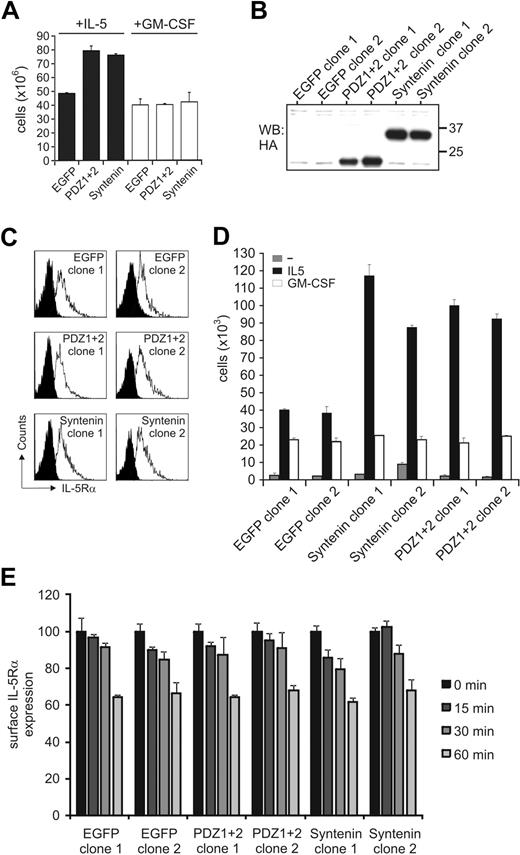
Cytokine-specific signal output within the GM-CSFR family may result from selective protein interactions mediated via the α-chains of these receptors. TF-1 cells endogenously express receptors for IL-5, IL-3, and GM-CSF, and these cytokines drive cell proliferation albeit with different concentration-dependent effects (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We generated polyclonal TF-1 cell lines ectopically expressing HA-tagged syntenin and HA-tagged mutant syntenin lacking its N-terminal domain (PDZ1+2) and stimulated with IL-5 and GM-CSF concentrations that would induce similar functional output. All transduced cells were marked by EGFP to facilitate cell sorting. Both syntenin and PDZ1+2-transduced cells showed enhanced proliferative responses to IL-5 but not to GM-CSF compared with mock-transduced cells expressing only EGFP, or the N-terminal domain or PDZ domain 1 of syntenin (Figure 2A and supplemental Figures 2-3). From lines expressing full-length syntenin or the PDZ tandem, 2 clones were generated for further analysis. Expression of the HA-tagged proteins in these clones is shown in Figure 2B and we found surface expression of IL-5Rα in these clones comparable (Figure 2C). The ectopically expressed proteins were 2- to 3-fold higher than endogenous levels (data not shown). Similar to the polyclonal cells, these clones were cytokine dependent, and showed enhanced proliferative responses to IL-5 but not to GM-CSF (Figure 2D, and supplemental Figure 4) despite these clones having similar IL-5Rα surface expression levels and IL-5–mediated internalization rates of IL-5Rα (Figure 2E). These data demonstrated that syntenin and its PDZ tandem can selectively modulate IL-5–mediated proliferation but do not affect IL-5Rα expression and internalization kinetics.
Syntenin selectively promotes IL-5–driven proliferation. (A) Stable polyclonal TF-1 cell lines were generated that ectopically express HA-tagged full-length syntenin, syntenin lacking its N-terminal domain (PDZ1+2), or EGFP as control. All lines were more than 95% EGFP positive. Viable cell numbers of 6-day cultures in either IL-5–supplemented (■) or GM-CSF–supplemented (□) medium are displayed. An average of 4 independent experiments is depicted (± SD). (B) Anti–HA-tagged Western blot indicating the presence of HA-tagged syntenin or PDZ1+2 in selected TF-1 cell–derived clones. Two clones per group are shown. (C) Expression of IL-5Rα for each subclone was analyzed by flow cytometry. Histograms of isotype-stained (black) or IL-5Rα–stained (white) cells are indicated. (D) Proliferative responses of selected clones to IL-5 or GM-CSF after 72 hours. Viable cells (live gate, propidium iodide negative) were counted by flow cytometry after culturing the cells for 3 days without cytokine, or after addition of IL-5 or GM-CSF. Mean of triplicate samples (± SD) is indicated for 2 control EGFP-clones, 2 clones transduced with syntenin, and 2 clones transduced with PDZ1+2. An average of 4 independent experiments is depicted (± SD). (E) Surface levels of clones expressing syntenin, PDZ1+2, or EGFP were incubated with IL-5 (10nM) for various time points and remaining surface expression was analyzed using flow cytometry. Data represent mean ± SD of triplicates.
Syntenin selectively promotes IL-5–driven proliferation. (A) Stable polyclonal TF-1 cell lines were generated that ectopically express HA-tagged full-length syntenin, syntenin lacking its N-terminal domain (PDZ1+2), or EGFP as control. All lines were more than 95% EGFP positive. Viable cell numbers of 6-day cultures in either IL-5–supplemented (■) or GM-CSF–supplemented (□) medium are displayed. An average of 4 independent experiments is depicted (± SD). (B) Anti–HA-tagged Western blot indicating the presence of HA-tagged syntenin or PDZ1+2 in selected TF-1 cell–derived clones. Two clones per group are shown. (C) Expression of IL-5Rα for each subclone was analyzed by flow cytometry. Histograms of isotype-stained (black) or IL-5Rα–stained (white) cells are indicated. (D) Proliferative responses of selected clones to IL-5 or GM-CSF after 72 hours. Viable cells (live gate, propidium iodide negative) were counted by flow cytometry after culturing the cells for 3 days without cytokine, or after addition of IL-5 or GM-CSF. Mean of triplicate samples (± SD) is indicated for 2 control EGFP-clones, 2 clones transduced with syntenin, and 2 clones transduced with PDZ1+2. An average of 4 independent experiments is depicted (± SD). (E) Surface levels of clones expressing syntenin, PDZ1+2, or EGFP were incubated with IL-5 (10nM) for various time points and remaining surface expression was analyzed using flow cytometry. Data represent mean ± SD of triplicates.
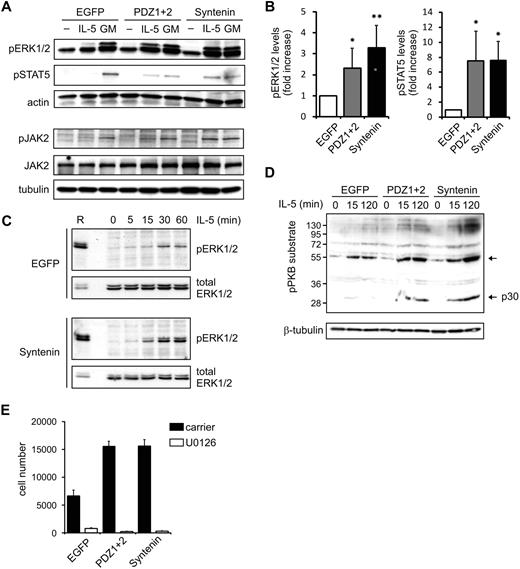
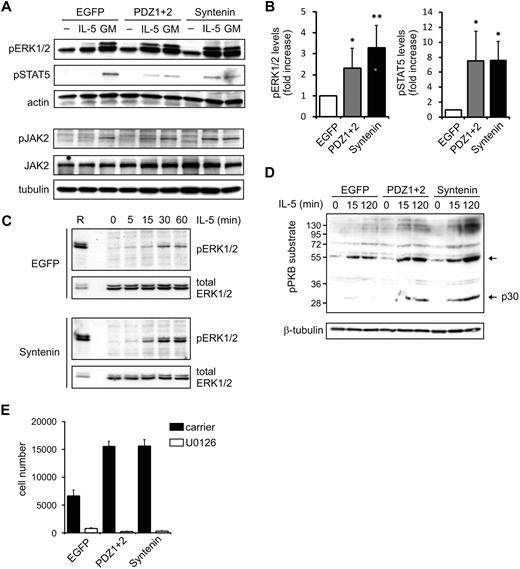
Next, we analyzed protein phosphorylation events elicited by both IL-5 and GM-CSF receptors in clones expressing HA-tagged (mutant) syntenin. The membrane-proximal regions of the α- and β-chains of these receptors associate with JAK kinases that initiate signaling upon ligand binding.4 Subsequent signaling activates STAT family members (the JAK-STAT pathway), and also the MAPK and PI-3K/PKB pathway, which all play important roles in cytokine-mediated cellular responses. TF-1 clones ectopically expressing HA-tagged (mutant) syntenin were stimulated with IL-5 or GM-CSF for 15 minutes after cytokine and serum starvation. These clones consistently showed increased levels of IL-5–induced phosphorylated ERK1 and ERK2 (MAPK family members) and STAT5 (Figure 3A, and supplemental Figure 5). These results were evident in 2 independent clones expressing syntenin or PDZ1+2 (data not shown) and were quantified in Figure 3B. Phospho-levels of the IL-5Rα–interacting protein JAK2 were also enhanced by syntenin expression (Figure 3A), whereas IL-5Rα–interacting Lyn kinase was not found regulated by IL-5 (data not shown).27,28 When we compared EGFP with PDZ1+2 or syntenin-transduced cells, we observed larger amplitudes of signaling rather than different signaling kinetics of these pathways (Figure 3C; a representative experiment comparing kinetics of pERK1/2 activation in EGFP or syntenin-transduced cells is shown). Similar results were observed in PDZ1+2-transduced cells, suggesting the NTD is not required. Different blots within a single experiment were comparable, as indicated by equal staining intensities of a reference sample that was shared between blots. Increased activation of the PI-3K/PKB pathway was suggested from analyses of phosphorylated PKB substrates after IL-5 stimulation (Figure 3D). Interestingly, among the presumed substrates of PKB, phosphorylation of a 30-kDa protein was profoundly affected by syntenin overexpression (Figure 3D; p30). To relate these signaling responses to the observed effects in IL-5–driven proliferation, we incubated cells with the specific ERK1/2-kinase inhibitor U0126. Proliferation was dramatically inhibited, indicating that pERK1/2 levels are critical for proliferation (Figure 3E). Together, these data indicate that syntenin selectively modulates signaling output of the IL-5R, but not of the GM-CSFR, and that these signals are important regulators of proliferation.
Syntenin increases IL-5–mediated signaling. (A) TF-1 cells ectopically expressing syntenin and PDZ1+2 were starved overnight in 0.5% serum and stimulated for 15 minutes with IL-5 or GM-CSF. Cell lysates were prepared, proteins quantified, and subsequent Western blots assessed for phosphorylated ERK1/2 (pERK1/2), phosphorylated STAT5 (pSTAT5), and actin in the top panel. A representative example of 4 independent experiments is shown. The bottom panel shows a representative example of Western blots assessed for phosphorylated and total JAK2, and tubulin. (B) pERK1/2 and pSTAT5 levels of 4 independent experiments as outlined in panel A were quantified using ImageJ software. Mean integrated intensity ± SD is indicated. *P < .05; **P < .01. (C) EGFP or ectopic syntenin-expressing cells were starved overnight and stimulated with IL-5 for indicated time points. Protein lysates were quantified for protein amount, and Western blots were prepared in duplicate and analyzed for pERK1/2 and total ERK levels. A reference sample (R) was included to facilitate comparison between blots; a lower amount of reference was loaded on the blots that were probed for total ERK. (D) Cells ectopically expressing EGFP, PDZ1+2, and syntenin were stimulated with IL-5 for indicated time points, and protein lysates were prepared and quantified. Subsequent Western blots were incubated with antibody recognizing phosphorylated PKB (pPKB) substrates and tubulin as loading control. One of 3 representative experiments is shown. (E) Cells ectopically expressing EGFP, PDZ1+2, and syntenin were cultured for 3 days in the presence of IL-5 and an inhibitor (U0126) of MEK1/2, the upstream kinases of ERK1/2. Viable cell numbers are depicted (average ± SD), and were determined by flow cytometry in 3 independent experiments.
Syntenin increases IL-5–mediated signaling. (A) TF-1 cells ectopically expressing syntenin and PDZ1+2 were starved overnight in 0.5% serum and stimulated for 15 minutes with IL-5 or GM-CSF. Cell lysates were prepared, proteins quantified, and subsequent Western blots assessed for phosphorylated ERK1/2 (pERK1/2), phosphorylated STAT5 (pSTAT5), and actin in the top panel. A representative example of 4 independent experiments is shown. The bottom panel shows a representative example of Western blots assessed for phosphorylated and total JAK2, and tubulin. (B) pERK1/2 and pSTAT5 levels of 4 independent experiments as outlined in panel A were quantified using ImageJ software. Mean integrated intensity ± SD is indicated. *P < .05; **P < .01. (C) EGFP or ectopic syntenin-expressing cells were starved overnight and stimulated with IL-5 for indicated time points. Protein lysates were quantified for protein amount, and Western blots were prepared in duplicate and analyzed for pERK1/2 and total ERK levels. A reference sample (R) was included to facilitate comparison between blots; a lower amount of reference was loaded on the blots that were probed for total ERK. (D) Cells ectopically expressing EGFP, PDZ1+2, and syntenin were stimulated with IL-5 for indicated time points, and protein lysates were prepared and quantified. Subsequent Western blots were incubated with antibody recognizing phosphorylated PKB (pPKB) substrates and tubulin as loading control. One of 3 representative experiments is shown. (E) Cells ectopically expressing EGFP, PDZ1+2, and syntenin were cultured for 3 days in the presence of IL-5 and an inhibitor (U0126) of MEK1/2, the upstream kinases of ERK1/2. Viable cell numbers are depicted (average ± SD), and were determined by flow cytometry in 3 independent experiments.
Syntenin knockdown decreases IL-5R output
To further confirm a role for syntenin in IL-5R function, we analyzed IL-5 responses in TF-1 cells after syntenin knockdown by short interference RNA (siRNA) and retrovirally transduced short hairpin RNA (shRNA). Transient knockdown of syntenin in TF-1 cells using 2 siRNAs that target different syntenin sequences (Figure 4A) resulted in reduced levels of phosphorylated ERK1/2 after IL-5 stimulation that were not observed with scrambled control siRNA (Figure 4B). GM-CSF–induced signals remained unchanged, indicating that βc-induced ERK1/2 signaling was intact. Syntenin-dependent IL-5–mediated proliferation was also analyzed in polyclonal TF-1 cells retrovirally infected with control shRNA or syntenin-targeting shRNA19 (Figure 4C). Viable cell numbers were determined after growth for 3 days in IL-5 or GM-CSF and expressed as percentage of control shRNA-treated cells (Figure 4D). We observed that syntenin-targeted shRNA consistently reduced cell yields in the presence of IL-5 but not GM-CSF. Taken together, these data strongly supported a role for syntenin in regulating IL-5R function.
Decreased IL-5R functions upon knockdown of syntenin in TF-1 cells. (A) Scrambled control siRNa and syntenin directed siRNA were electroporated into TF-1 cells using an Amaxa system. TF-1 cells recovered after 24 hours in the presence of IL-3 (cells were ∼ 90% trypan blue negative), and were starved for 4 hours before cells were stimulated with IL-5 for 15 minutes. Cells were lysed in Laemmli buffer, proteins content was quantified, and Western blots were stained for syntenin and tubulin as loading control. (B) pERK1/2 levels (± SD) were assessed by quantifying Western blot from 3 independent experiments as described in panel A using ImageJ software. (C) TF-1 cells were transduced with bicistronic constructs that express nonfunctional shRNA (control) or syntenin-targeting shRNA in combination with EGFP. EGFP cells were sorted beyond 95% purity and syntenin levels were assessed by Western blot. (D) Control and syntenin-targeting shRNA-expressing TF-1 cells were cultured for 3 days in the presence of IL-5 or GM-CSF. Viable cell numbers were determined by flow cytometry. Results depicted are from 2 experiments with 4 samples per group per experiment (± SD). *P < .05.
Decreased IL-5R functions upon knockdown of syntenin in TF-1 cells. (A) Scrambled control siRNa and syntenin directed siRNA were electroporated into TF-1 cells using an Amaxa system. TF-1 cells recovered after 24 hours in the presence of IL-3 (cells were ∼ 90% trypan blue negative), and were starved for 4 hours before cells were stimulated with IL-5 for 15 minutes. Cells were lysed in Laemmli buffer, proteins content was quantified, and Western blots were stained for syntenin and tubulin as loading control. (B) pERK1/2 levels (± SD) were assessed by quantifying Western blot from 3 independent experiments as described in panel A using ImageJ software. (C) TF-1 cells were transduced with bicistronic constructs that express nonfunctional shRNA (control) or syntenin-targeting shRNA in combination with EGFP. EGFP cells were sorted beyond 95% purity and syntenin levels were assessed by Western blot. (D) Control and syntenin-targeting shRNA-expressing TF-1 cells were cultured for 3 days in the presence of IL-5 or GM-CSF. Viable cell numbers were determined by flow cytometry. Results depicted are from 2 experiments with 4 samples per group per experiment (± SD). *P < .05.
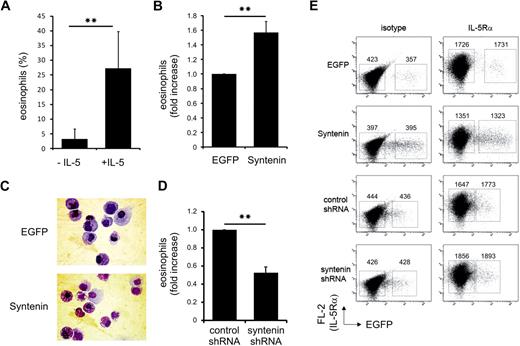
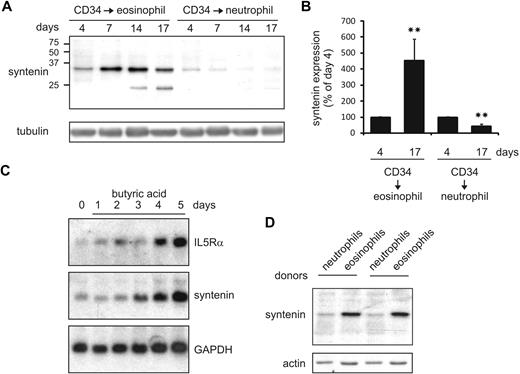
Syntenin is critical for eosinophil differentiation
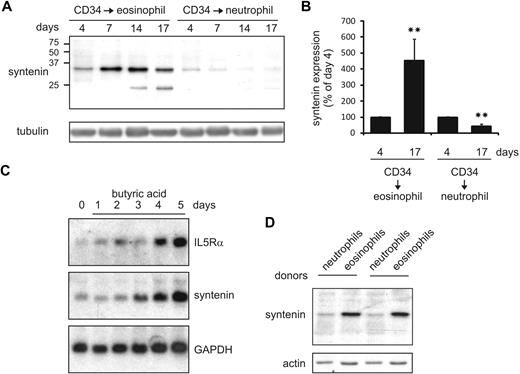
IL-5 selectively drives the production of eosinophils from multipotent myeloid progenitor cells.1,11,13 To investigate a potential role for syntenin in IL-5–driven eosinophilopoiesis, we cultured primary human cord blood–derived CD34+ cells in the presence of IL-3 and IL-5 to induce eosinophil differentiation. In these cultures, syntenin was strongly up-regulated as indicated by Western blot analysis (Figure 5A-B). IL-5Rα chain expression was concurrently increased in these cultures as indicated by flow cytometric analysis (data not shown). The syntenin-recognizing antibody also detected an approximately 22-kDa protein in eosinophil cultures that may be syntenin-2 isoform b based on predicted molecular weight and high similarity with the part of syntenin-1 to which the polyclonal antibody was raised. Conversely, syntenin was down-regulated when CD34+ cells were differentiated to neutrophils in the presence of G-CSF. In a second approach, we investigated IL-5Rα and syntenin mRNA levels in a commonly used model system of eosinophil differentiation that uses HL-60 cells precultured for 2 months at pH 7.7.29 When eosinophil differentiation was induced by addition of butyric acid, we observed that IL-5Rα and syntenin mRNA expression were both increased (Figure 5C). We also analyzed syntenin levels of various immune cells in peripheral blood and observed increased levels of syntenin in mature eosinophils compared with neutrophils (2 donors are shown in Figure 5D). Thus, in 2 independent eosinophil differentiation models, we observed that syntenin and IL-5Rα expression was concomitantly regulated, and we found high syntenin levels in mature eosinophils compared with neutrophils.
Syntenin expression is induced during eosinophil differentiation. (A) CD34+ cells were isolated from cord blood and maintained in FLT-3, SCF, IL-3, GM-CSF, and IL-5. At day 3, cells were transferred to medium containing IL-3 and IL-5 to induce eosinophil differentiation, or to G-CSF–supplemented medium to induce neutrophils. Protein lysates were prepared at the days indicated, and 30 μg of protein was loaded per lane. Blots were incubated with antibodies recognizing syntenin and tubulin as loading control. One of 4 representative Western blots is indicated. (B) Syntenin levels from 4 Western blots were quantified using ImageJ software and mean integrated density is displayed (± SD). **P < .01. (C) Northern blot analysis of HL-60 cells maintained at pH 7.7 for 2 months (HL-60 7.7) that were differentiated toward the eosinophilic lineage by 0.5 mM butyric acid (days of treatment are indicated). Total RNA (20 μg) was blotted and probed for IL-5Rα, syntenin, and GAPDH as loading control. (D) From 2 distinct donors, eosinophils and neutrophils were purified based on Ficoll centrifugation and CD16 selection (magnetic-activated cell sorting; Miltenyi Biotec). Thirty micrograms protein of whole-cell lysate was assessed by Western blotting for syntenin and actin levels.
Syntenin expression is induced during eosinophil differentiation. (A) CD34+ cells were isolated from cord blood and maintained in FLT-3, SCF, IL-3, GM-CSF, and IL-5. At day 3, cells were transferred to medium containing IL-3 and IL-5 to induce eosinophil differentiation, or to G-CSF–supplemented medium to induce neutrophils. Protein lysates were prepared at the days indicated, and 30 μg of protein was loaded per lane. Blots were incubated with antibodies recognizing syntenin and tubulin as loading control. One of 4 representative Western blots is indicated. (B) Syntenin levels from 4 Western blots were quantified using ImageJ software and mean integrated density is displayed (± SD). **P < .01. (C) Northern blot analysis of HL-60 cells maintained at pH 7.7 for 2 months (HL-60 7.7) that were differentiated toward the eosinophilic lineage by 0.5 mM butyric acid (days of treatment are indicated). Total RNA (20 μg) was blotted and probed for IL-5Rα, syntenin, and GAPDH as loading control. (D) From 2 distinct donors, eosinophils and neutrophils were purified based on Ficoll centrifugation and CD16 selection (magnetic-activated cell sorting; Miltenyi Biotec). Thirty micrograms protein of whole-cell lysate was assessed by Western blotting for syntenin and actin levels.
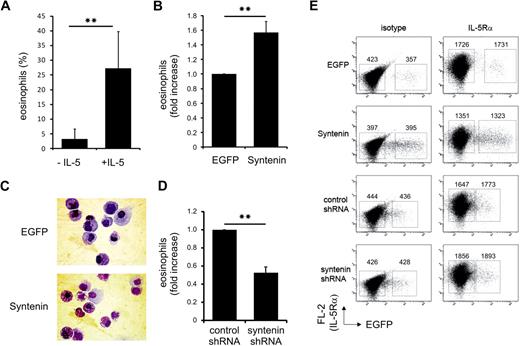
To investigate whether syntenin plays a critical role in regulating eosinophil differentiation, we modulated syntenin levels in primary CD34+ cells by retroviral transduction with full-length syntenin or syntenin-targeting shRNA. Eosinophil differentiation depends on IL-5 (Figure 6A), and we found ectopic expression of syntenin significantly increased eosinophil numbers (Figure 6B). Representative cytospins of these cultures are presented in Figure 6C. We did not observe different proliferation rates of EGFP and syntenin-transduced CD34+ progenitors (data not shown), which could be attributed to the presence of IL-3 in these cultures. CD34+ cells were transduced with control or syntenin-targeting shRNA to evaluate eosinophil differentiation after ablation of syntenin expression. We observed a clear reduction in eosinophil numbers upon transduction of syntenin-targeting shRNA (Figure 6D). Surface expression of IL-5Rα was unaffected by syntenin overexpression or knockdown (Figure 6E). Taken together, these data indicated that syntenin modulates IL-5–driven eosinophil differentiation in primary CD34+ progenitor cells.
Syntenin modulates eosinophil differentiation. (A) CD34+ cells were isolated from cord blood, and cultured for 3 days in FLT-3, SCF, IL-3, and GM-CSF before the medium was supplemented with IL-3 in the presence or absence of IL-5. Cells were transduced with EGFP control virus at days 1 and 2. At day 17, EGFP+ cells were sorted, cytospins were prepared, and cells were stained by May-Grünwald-Giemsa. Percentage of eosinophils was scored in 4 independent experiments using different donors (± SD). (B) Eosinophil differentiations of 3 independent donors upon transduction with EGFP control protein or syntenin and incubation with IL-5 as described in panel A. Eosinophil numbers after EGFP transduction were set at 100%, and the fold increase upon syntenin transduction was calculated per donor (± SD). (C) Representative cytospins at day 17 of EGFP or syntenin-transduced CD34+ cells that were differentiated toward eosinophils. EGFP-positive cells were sorted and stained by May-Grünwald-Giemsa. (D) CD34+ cells were transduced with retroviral vectors expressing control shRNA or syntenin-targeting shRNA and differentiated toward eosinophils. At day 17, fold increase of the percentage of eosinophils for each donor (n = 3, data show average ± SD) after control shRNA transductions (set at 100%) or syntenin-targeting shRNA transduction was determined. **P < .01. (E) Surface IL-5Rα expression of primary CD34+ cells transduced with EGFP or syntenin (3 donors) or control or syntenin-targeting shRNA (2 donors). Eosinophil cultures were analyzed at day 14 for IL-5Rα expression by flow cytometry (PE-channel). Nontransduced and transduced cells were indicated by EGFP transduction and IL-5Rα staining was compared with isotype staining. Numbers represent geometric mean fluorescent intensities of the boxed region in the PE channel. Identical results were obtained for all donors and 1 representative sample is shown.
Syntenin modulates eosinophil differentiation. (A) CD34+ cells were isolated from cord blood, and cultured for 3 days in FLT-3, SCF, IL-3, and GM-CSF before the medium was supplemented with IL-3 in the presence or absence of IL-5. Cells were transduced with EGFP control virus at days 1 and 2. At day 17, EGFP+ cells were sorted, cytospins were prepared, and cells were stained by May-Grünwald-Giemsa. Percentage of eosinophils was scored in 4 independent experiments using different donors (± SD). (B) Eosinophil differentiations of 3 independent donors upon transduction with EGFP control protein or syntenin and incubation with IL-5 as described in panel A. Eosinophil numbers after EGFP transduction were set at 100%, and the fold increase upon syntenin transduction was calculated per donor (± SD). (C) Representative cytospins at day 17 of EGFP or syntenin-transduced CD34+ cells that were differentiated toward eosinophils. EGFP-positive cells were sorted and stained by May-Grünwald-Giemsa. (D) CD34+ cells were transduced with retroviral vectors expressing control shRNA or syntenin-targeting shRNA and differentiated toward eosinophils. At day 17, fold increase of the percentage of eosinophils for each donor (n = 3, data show average ± SD) after control shRNA transductions (set at 100%) or syntenin-targeting shRNA transduction was determined. **P < .01. (E) Surface IL-5Rα expression of primary CD34+ cells transduced with EGFP or syntenin (3 donors) or control or syntenin-targeting shRNA (2 donors). Eosinophil cultures were analyzed at day 14 for IL-5Rα expression by flow cytometry (PE-channel). Nontransduced and transduced cells were indicated by EGFP transduction and IL-5Rα staining was compared with isotype staining. Numbers represent geometric mean fluorescent intensities of the boxed region in the PE channel. Identical results were obtained for all donors and 1 representative sample is shown.
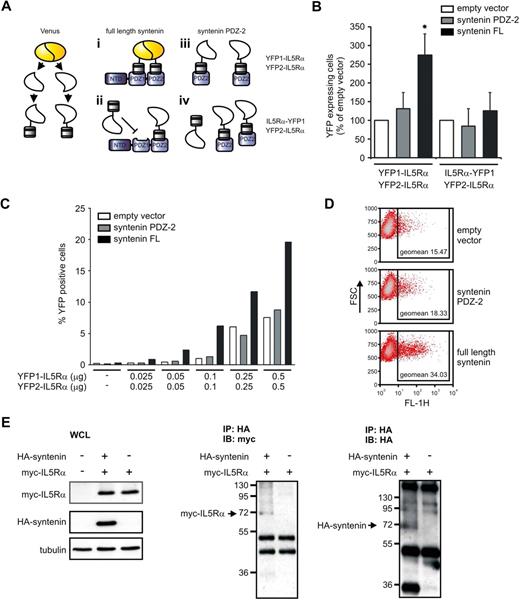
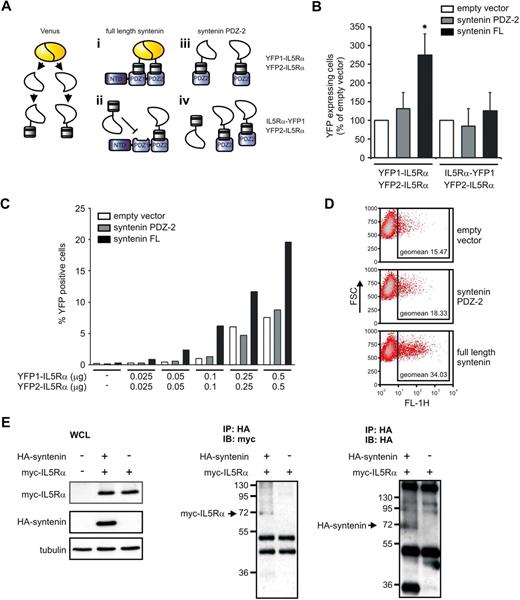
Syntenin binds multiple IL-5Rα chains
These observations provide compelling evidence that syntenin modulates IL-5R signaling and eosinophil differentiation, but do not directly address the molecular mechanism. The active IL-5R signaling complex likely forms a quaternary complex that consists of multiple IL-5Rα and βc chains as was observed for the GM-CSF receptor complex. Recent work indicated that GM-CSF bound to α and βc forms hexamer and dodecamer structures of 2:2:2 and 4:4:4 stoichiometry, respectively (cytokine:α:β).4,30,31 Importantly, IL-5 could be modeled in these structures. We hypothesized that syntenin may play a role in organizing and stabilizing an IL-5R signaling complex by binding to multiple IL-5Rα chains because both PDZ1 and PDZ2 can independently interact with an IL-5Rα–derived peptide.32 We first tested whether syntenin can bind multiple IL-5Rαs using a protein complementation assay (PCA) that uses Venus, an improved yellow fluorescent protein (YFP), as readout.33,34 In this PCA, nonfluorescent parts of YFP (YFP1 and YFP2) are fused to intracellular IL-5Rα domains, and we assessed whether coexpression of syntenin could facilitate YFP fluorescence (the various experimental conditions are schematically represented in Figure 7A). HEK-293 cells were transfected with increasing concentrations of YFP1–IL-5Rα and YFP2–IL-5Rα in the presence of full-length syntenin, PDZ domain 2 only, or empty vector. Cotransfection of syntenin or its PDZ tandem, but not a single PDZ2 domain or empty vector, resulted in a consistent increase of fluorescent cells as indicated by flow cytometry (Figure 7B and supplemental Figure 6). When the YFP1 fragment was fused to the C-terminus of IL-5Rα (IL-5Rα–YFP1), syntenin did not significantly increase YFP fluorescence, indicating that syntenin-enhanced fluorescence depends on a free C-terminal IL-5Rα sequence as would be predicted (Figure 7B).17 Syntenin-enhanced fluorescence was also related to YFP1–IL-5Rα and YFP2–IL-5Rα levels in a dose-dependent manner (Figure 7C). We also observed higher fluorescent signals (geometric mean intensity of the positive cells) when cells were cotransfected with syntenin (Figure 7D). We did not observe any fluorescence upon expression of either YFP1 or YFP2 fusion proteins alone (data not shown). To further demonstrate the ability of syntenin to bind multiple IL-5Rα chains, we expressed YFP2–myc–IL-5Rα with or without HA-syntenin in HEK293 cells and performed immunoprecipitations for HA in the presence of a chemical cross-linker to stabilize IL-5Rα–syntenin complexes (Figure 7E). Lysates showing myc–IL-5Rα (∼ 17.5 kDa) and HA-syntenin (∼ 35 kDa) expression are shown in the left panel. We observed syntenin-dependent coprecipitation of myc–IL-5Rα complexes around 70 kDa corresponding to 1 syntenin molecule bound to 2 myc–IL-5Rα proteins as shown in Figure 7E middle panel. In the same samples, we also observed HA reactivity around 70 kDa, suggesting that syntenin was part of the complex (Figure 7E right panel). Together, these data suggest that syntenin can bind multiple IL-5Rα chains and thus support a model in which syntenin can participate in the formation and/or stabilization of an IL-5–induced oligomeric IL-5Rαβ signaling complex.
Syntenin binds multiple IL-5Rα chains. (A) Schematic representation of the experimental design of the PCA. Venus, an improved version of yellow fluorescent protein (YFP), was fragmented into nonfunctional parts that were fused to the N-termini of the intracellular domains of IL-5Rα (YFP1–IL-5Rα and YFP2–IL-5Rα). When both of the fusion products bind syntenin, the nonfunctional Venus parts regain fluorescence. (i) As a control, expression of the fusion products with a single PDZ domain of syntenin should not result in functional restoration of the Venus protein. (ii) As an additional control, we fused YFP-1 to the C-terminus of IL-5Rα (IL-5Rα–YFP1) instead of its N-terminus. Because syntenin binds the C-terminus of IL-5Rα, no additional fluorescence is expected upon full-length syntenin or PDZ2 cotransfection (iii and iv, respectively). (B) HEK293 cells were transiently transfected with YFP1–IL-5Rα and YFP2–IL-5Rα together with empty vector, or syntenin's second PDZ domain (PDZ2), or full-length syntenin (left panel). The percentage of positive cells was determined by flow cytometry. At right, similar conditions were assayed but an IL-5Rα–YFP1 instead of YFP1–IL-5Rα fusion product was used. Empty vector transfections per experiments were set at 100%. Data depict 3 independent experiments (± SD). *P < .05. (C) YFP1–IL-5Rα and YFP2–IL-5Rα dose-dependent increase of fluorescence in the presence of full-length syntenin. Cells were transfected with fixed amounts of empty vector, syntenin PDZ2 or full-length syntenin (each 0.5 μg), and increasing concentrations of YFP1–IL-5Rα and YFP2–IL-5Rα. Cells were analyzed 48 hours after transfection for YFP fluorescence by flow cytometry. Percentage of YFP-fluorescent cells is indicated. One representative example of 2 identical experiments is indicated. (D) Representative dot plots of cells transfected with empty vector, syntenin PDZ2 or full-length syntenin (0.5 μg), together with 0.5 μg of YFP1–IL-5Rα and YFP2–IL-5Rα. Forward scatter is plotted against YFP fluorescence in the FL-1 channel. Geometric mean fluorescent intensities of the positive cells are indicated in the squares. (E) Immunoprecipitation (IP) of syntenin–IL-5Rα complexes from transfected HEK293 cells in the presence of the chemical cross-linker DSP. The left panels indicate expression of the myc–IL-5Rα (17.5 kDa) and HA-tagged syntenin (35 kDa) in whole-cell lysates (WCLs). The middle panel shows HA immunoprecipitates analyzed by nonreducing SDS-PAGE and myc-immunoblotting (IB), whereas the right panel shows HA immunoreactivity of the same samples blotted in duplicate.
Syntenin binds multiple IL-5Rα chains. (A) Schematic representation of the experimental design of the PCA. Venus, an improved version of yellow fluorescent protein (YFP), was fragmented into nonfunctional parts that were fused to the N-termini of the intracellular domains of IL-5Rα (YFP1–IL-5Rα and YFP2–IL-5Rα). When both of the fusion products bind syntenin, the nonfunctional Venus parts regain fluorescence. (i) As a control, expression of the fusion products with a single PDZ domain of syntenin should not result in functional restoration of the Venus protein. (ii) As an additional control, we fused YFP-1 to the C-terminus of IL-5Rα (IL-5Rα–YFP1) instead of its N-terminus. Because syntenin binds the C-terminus of IL-5Rα, no additional fluorescence is expected upon full-length syntenin or PDZ2 cotransfection (iii and iv, respectively). (B) HEK293 cells were transiently transfected with YFP1–IL-5Rα and YFP2–IL-5Rα together with empty vector, or syntenin's second PDZ domain (PDZ2), or full-length syntenin (left panel). The percentage of positive cells was determined by flow cytometry. At right, similar conditions were assayed but an IL-5Rα–YFP1 instead of YFP1–IL-5Rα fusion product was used. Empty vector transfections per experiments were set at 100%. Data depict 3 independent experiments (± SD). *P < .05. (C) YFP1–IL-5Rα and YFP2–IL-5Rα dose-dependent increase of fluorescence in the presence of full-length syntenin. Cells were transfected with fixed amounts of empty vector, syntenin PDZ2 or full-length syntenin (each 0.5 μg), and increasing concentrations of YFP1–IL-5Rα and YFP2–IL-5Rα. Cells were analyzed 48 hours after transfection for YFP fluorescence by flow cytometry. Percentage of YFP-fluorescent cells is indicated. One representative example of 2 identical experiments is indicated. (D) Representative dot plots of cells transfected with empty vector, syntenin PDZ2 or full-length syntenin (0.5 μg), together with 0.5 μg of YFP1–IL-5Rα and YFP2–IL-5Rα. Forward scatter is plotted against YFP fluorescence in the FL-1 channel. Geometric mean fluorescent intensities of the positive cells are indicated in the squares. (E) Immunoprecipitation (IP) of syntenin–IL-5Rα complexes from transfected HEK293 cells in the presence of the chemical cross-linker DSP. The left panels indicate expression of the myc–IL-5Rα (17.5 kDa) and HA-tagged syntenin (35 kDa) in whole-cell lysates (WCLs). The middle panel shows HA immunoprecipitates analyzed by nonreducing SDS-PAGE and myc-immunoblotting (IB), whereas the right panel shows HA immunoreactivity of the same samples blotted in duplicate.
Discussion
Although the receptors for IL-5, IL-3, and GM-CSF all recruit βc for signal transmission, functional output of these cytokines is clearly distinct, indicating that additional information is relayed in parallel with βc-induced signals.6 However, little is known about the molecular pathways that control signal specificity. We previously found the PDZ domain containing adapter protein syntenin to specifically bind the IL-5Rα chain.17 Adapter proteins are noncatalytic proteins that consist of multiple protein-interaction domains that serve to spatially and temporarily regulate the assembly of protein complexes.3 We now present data that syntenin specifically modulates IL-5–mediated functions in CD34+ hematopoietic progenitor cells by increasing signal output of IL-5R. To our knowledge, syntenin is the first adapter protein shown to modulate lineage-choice decisions during myelopoiesis. These data demonstrate that cytokine-receptor output can be regulated by adapters selectively interacting with the α-chain of cytokine receptors.
Addition of IL-5 induces internalization of IL-5Rαβ, which are degraded by the proteasome and lysosome.23,24 We found EGFP-tagged syntenin in TF-1 cells enriched at the plasma membrane and early endosomes as concluded from colabeling with transferrin receptors, CD63 and CD107a (Figure 1). IL-5Rα colocalizes at these sites with syntenin independent of IL-5 stimulation. In agreement, syntenin has previously been shown to localize to the plasma membrane where it modulates internalization kinetics of CD63 and Delta 1, a Notch ligand.19,35 In other cells, syntenin-positive endosomes colocalized with other early endosomal markers such as Rab5 and Rab7.36 A role for syntenin at slow recycling endosomes was also suggested by colocalization with Arf-6 where syntenin regulates recycling of syndecans.37 However, we did not find differences in IL-5Rα cell-surface levels upon increased syntenin expression, and found no changes in IL-5Rα internalization after IL-5 stimulation (Figures 2C and 6E). The data presented here suggest syntenin accompanies IL-5Rα during the early steps of ligand binding, and internalization toward early endosomes.
We found that syntenin enhanced IL-5–dependent cellular output in TF-1 cells, as indicated by ectopic expression and knockdown of syntenin (Figures 2,Figure 3–4). In these cells, the PDZ tandem and full-length syntenin induced comparable effects, suggesting a model in which the tandem alone can enhance IL-5Rαβ function independent of syntenin's NTD. We have previously described that SOX4 can associate with syntenin's NTD, however, we were unable to detect SOX4 expression in TF-1 cells (data not shown). Our data indicate that the tandem PDZ domain of syntenin is important for modulation of IL-5R signal output independent of the NTD. However, the activation of specific signal transduction pathways by IL-5Rα/syntenin may be regulated by unique NTD-interacting proteins such as SOX4 and is not mutually exclusive with our current data.
Expression of both IL-5Rα and syntenin is up-regulated during IL-5–driven eosinophil differentiation, as illustrated by cultures of primary human CD34+ cells and HL-60 cells (Figure 5). Conversely, syntenin is down-regulated during neutrophil differentiation. How signals initiated by the cytokines (IL-5 for eosinophils, and G-CSF for neutrophils) regulate syntenin expression is unclear. IL-5 signaling may act directly on the syntenin promoter or indirectly by up-regulating transcription factors that activate a positive feedback mechanism. Syntenin promoter analysis by Tfsitescan (Institute for Transcriptional Informatics, http://www.ifti.org) revealed a STAT5b responsive site, suggesting that IL-5 may indeed directly influence syntenin transcription. In addition, 2 PU.1 sites, a C/EBPα site, and, importantly, a GATA-1 site were also present, suggesting that transcription factors that play crucial roles during eosinophil differentiation may reinforce the IL-5R/syntenin pathway.38,39 In an analogous fashion, transcription factors that control T helper cell differentiation have been shown to stimulate expression of factors that control their own activation.40-43 We propose a model in which IL-5 via IL-5Rα/syntenin stimulates key eosinophil transcription factors, which in turn reinforce IL-5Rα and syntenin expression as part of a positive feedback mechanism. In agreement, addition of IL-5 or allergens increases IL-5 responsiveness and IL-5R expression of bone marrow cells in vitro and in vivo.44-46 Coregulation of IL-5Rα and syntenin could thus reinforce IL-5–specific signaling to promote eosinophil commitment of differentiating myeloid progenitor cells.
Ligand-receptor interactions in biologic systems are highly complex as indicated by the recently published crystal structure of GM/GM-CSFRα/βc that forms a large dodecameric complex upon ligand binding.31,47 In this study, and others, it has been suggested that the active IL-5R complex also consists of multiple IL-5Rα chains.4 Because syntenin has 2 PDZ domains that can bind IL-5R–derived peptides with relatively similar affinity,32 we reasoned that syntenin-enhanced IL-5R output could result from binding of syntenin to multiple IL-5Rα chains at the same time, which may lead to the stabilization of an active IL-5Rαβ signaling complex. Using a protein fragment complementation assay, we found syntenin could indeed drive IL-5Rα complex formation, which likely involves 1 syntenin molecule binding to 2 IL-5Rα chains as suggested from cross-linking experiments (Figure 7). It is possible that IL-5 orients the intracellular domains of IL-5Rα in such a way that these will optimally fit the tandem PDZ domains of syntenin. If IL-5 induces a similar receptor complex as observed for GM-CSF, the intracellular tails of IL-5Rα would be separated by approximately 30 Å, which corresponds to the distance of the peptide-binding pockets of PDZ1 and PDZ2 in syntenin.31,48,49 Furthermore, phosphoinositol lipids, especially phosphatidyl-inositol 4,5 bisphosphate, have high affinity for PDZ1 and may regulate IL-5Rα binding by competing with IL-5Rα for binding to PDZ1.50 IL-5–induced signaling modulates local phosphoinositol lipid composition (eg, by PI-3K activation), which could very well regulate PDZ1 accessibility for proteins such as IL-5Rα and syntenin-dependent IL-5R output.
The IL-5Rα-syntenin interaction may also play a crucial role in the development of a specific B-cell lineage termed B-1 cells.11,12,16 B-1 cells are major producers of natural antibodies and develop mainly during fetal life, whereas conventional B-2 cells develop in adult life.51 We previously reported that syntenin is required for IL-5–induced activation of SOX4, an HMG-box–containing transcription factor that binds to the N-terminal domain of syntenin.17 Interestingly, the carboxy-terminal region of IL-5Rα has been shown to mediate IgM to IgG1 class switching and B-1 B-cell development, and reconstitution of hematopoiesis by SOX4−/− fetal liver cells shows impaired pro–B-cell development.52 However, a role for IL-5Rα–syntenin–SOX4 axis in B-1 B-cell development has not been addressed directly.
Here, we established that endogenous IL-5R and syntenin functionally interact in TF-1 cells and primary CD34+ hematopoietic progenitor cells. We show for the first time that an adapter protein that binds IL-5Rα plays an important role in eosinophil differentiation by specifically regulating signal output of the IL-5R. Our data indicate that the PDZ tandem of syntenin regulates signal strength of the IL-5R. Association of intracellular effector proteins with syntenin may result in IL-5–specific signaling pathways that drive eosinophil differentiation. Identification of such factors should provide further insight into the molecular mechanisms underlying cytokine-receptor specificity. Taken together, our data demonstrate syntenin to be important for IL-5R function and eosinophilopoiesis, and support a model in which signal output within the GM-CSFR family can be modulated through α-chain–interacting proteins. The development of pharmacologic agents that specifically disrupt IL-5Rα–syntenin interaction may by themselves or in combination with anti–IL-5 therapy prove useful in a variety of IL-5–dependent eosinophilic diseases including allergy and idiopathic hypereosinophilic syndrome.44,53,54
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We express our gratitude to Drs S. W. Michnick and I. Remy (both Université de Montréal) for providing the YFP-PCA constructs, Drs S. Estrach and F. M. Watt (Cancer Research UK Cambridge Research Institute) for providing the retroviral shRNA constructs, and Dr X. Zhang (National Center of Biomedical Analysis) for providing DsRed-tagged syntenin cDNA. Furthermore, we thank R&D Systems for generously providing TF-1 cells and Dr E. van Binsbergen (Department of Medical Genetics, University Medical Center Utrecht) and L. A. Houben (Department of Pulmonary Diseases, University Medical Center Utrecht) for technical assistance.
J.M.B. was supported by a grant from the Dutch Scientific Organization (NWO 917.36.316).
Authorship
Contribution: J.M.B. designed, performed, and analyzed experiments and wrote the paper; L.P.V. and N.G. performed and analyzed experiments; and P.J.C. designed and analyzed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul J. Coffer, Wilhelmina Childrens Hospital, University Medical Centre Utrecht, Lundlaan 6, Utrecht 3584 EA, The Netherlands; e-mail: p.j.coffer@umcutrecht.nl.