Abstract
Adenosine deaminase deficiency is a disorder of purine metabolism leading to severe combined immunodeficiency (ADA-SCID). Without treatment, the condition is fatal and requires early intervention. Haematopoietic stem cell transplantation is the major treatment for ADA-SCID, although survival following different donor sources varies considerably. Unlike other SCID forms, 2 other options are available for ADA-SCID: enzyme replacement therapy (ERT) with pegylated bovine ADA, and autologous haematopoietic stem cell gene therapy (GT). Due to the rarity of the condition, the lack of large scale outcome studies, and availability of different treatments, guidance on treatment strategies is limited. We have reviewed the currently available evidence and together with our experience of managing this condition propose a consensus management strategy. Matched sibling donor transplants represent a successful treatment option with high survival rates and excellent immune recovery. Mismatched parental donor transplants have a poor survival outcome and should be avoided unless other treatments are unavailable. ERT and GT both show excellent survival, and therefore the choice between ERT, MUD transplant, or GT is difficult and dependent on several factors, including accessibility to the different modalities, response of patients to long-term ERT, and the attitudes of physicians and parents to the short- and potential long-term risks associated with different treatments.
Introduction
Adenosine deaminase (ADA) deficiency is a rare inherited disorder of purine metabolism characterized by the accumulation of metabolic substrates that lead to abnormalities of immune system development and function and a variety of systemic defects. The initial and most devastating presentation of the disease is the result of the immune defects; affected infants characteristically present with severe opportunistic infection and failure to thrive and an immunologic profile consistent with severe combined immunodeficiency (ADA-SCID). Without treatment, the condition is fatal in the first year of life and therefore necessitates early intervention. Allogeneic hematopoietic stem cell transplantation (HSCT) has long been considered the mainstay of treatment of ADA-SCID. However, unlike other SCID forms, 2 other treatment options are available for ADA-SCID, namely, enzyme replacement therapy (ERT) with pegylated bovine ADA (Pegadamase, Adagen, or PEG-ADA) and autologous HSC gene therapy (GT).
The availability of different treatment modalities presents an opportunity for improved patient care but also presents difficulties in deciding on the specific choice of treatment for individual patients. Making the correct choice is further complicated by the fact that ADA deficiency is not purely an immune defect, unlike other SCID forms, and the systemic manifestations, which can be of major clinical consequence, must also be managed. The rarity of the condition (estimated at anything from 1:200 000 to 1:1 million births) means that any one center has access to only small numbers of patients precluding large-scale prospective studies from which formal and robust outcome data can be accrued. For these reasons, we have gathered experts with experience in management of patients using the different treatment modalities to collect and review the available retrospective data and to construct a consensus management strategy that may be of use to physicians managing this difficult patient population.
ADA-SCID
SCID arises from a variety of molecular defects that have profound effects on lymphocyte development and function, including defects in the lymphocyte-specific signaling molecules (common γ chain, JAK-3, and IL-7 receptor α) and in molecules that control rearrangement of the T-cell receptor and immunoglobulin genes (RAG-1/2, Artemis, DNA ligase IV, DNA-PKcs, Cernunnos/XLF) or signaling through the pre-T-cell receptor (CD3δ, CD3ϵ, CD3ζ, and CD45).1 ADA-deficient SCID accounts for approximately 10% to 20% of all cases of SCID and was the first form of SCID in which the underlying defect was identified.2
ADA is a purine salvage enzyme expressed in all tissues of the body and catalyzes the deamination of deoxydenosine (dAdo) and adenosine (Ado) to deoxyinosine and inosine, respectively.3 The absence of ADA results in accumulation of dAdo in both intracellular and extracellular compartments. Within cells, conversion of dAdo by deoxycytidine kinase (dCydK) to deoxyadenosinetrisphosphate (dATP) leads to intracellular expansion of the dATP pool. The buildup of both dATP and dAdo has deleterious effects on lymphocyte development and function and is the major cause of the immunologic defects. Studies on both ADA-deficient murine and human models show that dATP inhibits ribonucleotide reductase, an enzyme necessary for DNA replication and repair,4 induces apoptosis in immature thymocytes,5 and interferes with terminal deoxynucleotidyl transferase activity, thereby limiting V(D)J recombination and antigen receptor diversity.6 dAdo accumulation inactivates the enzyme S-adenosylhomocysteine hydrolase7 and results in inhibition of transmethylation reactions necessary for effective lymphocyte activation. Elevated levels of Ado, acting through cell-surface G protein–coupled receptors, may contribute to immune dysfunction and pulmonary inflammation associated with ADA deficiency. However, the profound and relatively selective lymphopenia of ADA deficiency may be best explained by the expression pattern of ADA, which is highest in the thymus as a result of high lymphocyte turnover8,9 and also by increased expression of dCydK in lymphocytes, which increases dATP accumulation from dAdo in immune cells more than in other tissues.10
The ubiquitous nature of ADA expression, however, does lead to a number of significant nonimmunologic defects. ADA-SCID patients have costochondral abnormalities and skeletal dysplasias,11 neurologic deficits involving motor function,12 bilateral sensorineuronal deafness,13 and hepatic dysfunction.14 ADA-SCID patients show defects in cognitive and behavioral function despite correction of immunologic abnormalities after bone marrow transplantation,15 thus highlighting the systemic nature of the disease. Nonimmunologic manifestations are also found in ADA-deficient mice, which exhibit hepatocyte degeneration, pulmonary and intestinal defects,16-18 and neurologic abnormalities19 with dATP and dAdo accumulation and S-adenosylhomocysteine hydrolase inhibition in affected tissues.
Management of ADA deficiency
When considering management of ADA-SCID, it is important initially to consider the principles for successful therapy. The underlying defect is an enzyme deficiency that leads to the buildup of toxic metabolites, which in turn have an effect on different organ systems, most notably the immune system. It is still not entirely clear whether lymphocytes from ADA-deficient patients are intrinsically abnormal or whether the defects seen are secondary to the effect of dATP and dAdo accumulation. In addition, accumulation of toxic metabolites may also interfere with thymic stroma development, maturation, and function, resulting in impaired ability to support T-cell development. Nevertheless, the principle remains that enzyme delivery is key to the successful detoxification of metabolic substrates and promotion of immune recovery. Enzyme delivery can therefore take the form of allogeneic wild-type cells, gene-modified autologous cells, or exogenous direct ERT. All 3 forms of therapy have been shown to promote effective immune recovery, and the challenge remains to examine the available evidence and to draw up a treatment hierarchy that may be used when considering patients presenting with different clinical scenarios and with different donor options.
HSCT
HSCT is the treatment choice that is most widely available to most physicians and transplantation centers. To date, there have been no formal data on the outcome of transplantation for ADA-SCID alone. The most notable SCID transplantation outcome papers have reported survival figures for all forms of SCID, and extensive data on ADA-SCID transplantation outcome and completeness of immune recovery have not been readily available. For instance, in the largest series of 475 SCID patients form the European SCETIDE database reported by Antoine et al,20 51 ADA patients were included and the study documented a 3-year survival of 81% for human leukocyte antigen (HLA)–matched transplantations and 29% for HLA-mismatched transplantations with no further information on the degree of immune recovery.20 Only 4 unrelated donor transplantations were reported in this series, and no outcome data on these patients were presented. Similarly in a 2-center study documenting outcome on 94 SCID patients, 6 ADA-SCID patients were included.21 The deficiency in formal outcome data has limited the ability to make informed choices regarding transplantation. This is further compounded by anecdotal physician experience that ADA patients have more difficulty with transplants, especially from unrelated and haploidentical donors, possibly because of the need for conditioning and the underlying metabolic nature of the condition.
For these reasons, a retrospective study was initiated to gather data on ADA transplantation outcome. The patients recruited into this study do include those analyzed in the previous reports.20,21 The preliminary results of the study were presented at the European Blood and Marrow Transplantation conference in Hamburg (2006),22 and more patients have since been added. Interim analyses of the data were presented at European Blood and Marrow Transplantation 2009 in Goteborg and highlight the following: Data from a total of 87 patients undergoing 97 transplantation procedures were recruited from European and North American centers, and the survival outcome is presented in Figure 1. The overall 1-year survival after matched sibling and matched family donor transplantations (often available because of the high incidence of consanguineous pedigrees) was 87% and 88%, respectively. Survival after fully matched unrelated donor transplantations was 67% (n = 11); in contrast, survival after mismatched unrelated or mismatched family donors (mainly parental haploidentical donors) was 29% and 43%, respectively, with the majority of deaths in the first few months after transplantation. The highly successful outcome in matched sibling and family donor transplantations is most probably the result of the absence of any chemotherapeutic regimen, and this is confirmed by univariate analysis. Interestingly, there was no difference between patients undergoing reduced-intensity conditioning (n = 8, survival = 50%) and those undergoing fully myeloablative conditioning (n = 38, survival = 50%). In addition, there was no survival benefit for patients who had received PEG-ADA to stabilize their clinical condition before HSCT. Data on immune recovery suggest that the large majority of patients who survive transplantation normalize absolute lymphocyte and T-cell numbers. Furthermore, of 38 patients in whom data were available, 35 were able to discontinue immunoglobulin replacement therapy, including 24 of 26 undergoing unconditioned sibling donor transplantations, suggesting that immune recovery is relatively complete in the majority of surviving patients. Similarly, patients are well detoxified after transplantation with marked reduction of dATP levels to a mean of approximately 100 μM, which, although not normal, represents an approximately 1-log reduction from levels at diagnosis.
Survival of patients receiving HSCT for ADA-SCID (preliminary results from an international multicenter retrospective study).
Survival of patients receiving HSCT for ADA-SCID (preliminary results from an international multicenter retrospective study).
The poor outcome in mismatched donor transplantations may be attributable to the vulnerability of patients to the toxicities associated with cytoreductive chemotherapy, and it has been argued that an alternative approach would be to infuse T cell–depleted stem cells without any chemotherapy. Data available from a single North American center suggest, however, that this may not necessarily improve disease-free survival (DFS) outcome. Of 19 patients undergoing such procedures, 14 survived (73%); but of these, only 7 were successfully engrafted (DFS = 37%), with the remainder rejecting the unconditioned transplant.22 Similarly, in the HSCT data collected recently, of 4 fully matched unrelated donor transplantations performed without conditioning, 2 patients did not engraft effectively, suggesting that conditioning is necessary in all scenarios other than a fully matched sibling or family donor.
The immunologic recovery after transplantation on the available data appears to be well maintained even after 10 years or longer in some patients. The prognosis for nonimmunologic manifestations is, however, not as good. Studies on cognitive and behavioral abnormalities highlight a high incidence of cognitive deficits with a mean IQ approximately 2 SDs below the population normal and behavioral problems characterized by hyperactivity and attention deficit.15,23 There is also a high incidence of audiologic defects, typically bilateral high-frequency sensorineuronal hearing loss.24 These abnormalities do not correlate with any specific type of transplantation, the conditioning regimen, degree of donor cell engraftment, or metabolite levels after HSCT. However, IQ was shown to correlate with the level of dATP (and by inference the degree of metabolic derangement) at the time of diagnosis, which may indicate that irreversible damage had occurred in the early neonatal period. At present, therefore, it is not possible to make donor or conditioning choices to improve nonimmunologic transplantation outcome after transplantation.
A number of other questions are raised with regard to transplantation management. One issue that arises is the role of ERT before or after HSCT. It could be argued that the use of ERT before transplantation may promote endogenous T-cell recovery, thereby inhibiting donor cell engraftment, or that ERT-mediated detoxification may remove the survival advantage of allogeneic cells. However, this must be balanced against the fact that ERT offers rapid metabolic detoxification, which can improve the clinical well-being of the child in terms of weight gain and nutrition before HSCT. Guided by our collective clinical experience and in the absence of any evidence to suggest that PEG-ADA is detrimental to transplantation outcome, we would recommend starting PEG-ADA before transplantation in any child when there is probably a long wait to find a donor or in any child who is clinically unwell, especially those with respiratory compromise and nutritional status. The role of PEG-ADA after a partially successful transplantation to promote immune recovery or indeed to ameliorate nonimmunologic problems has not been tested in any formal setting, and even anecdotal reports are not available.
The data on HSCT outcome (in “HSCT”) may help in guiding decision-making with respect to transplantation strategies. Certainly, a fully matched sibling or family donor transplantation is associated with a considerable degree of success in terms of both survival and sustained immune recovery and would be the definitive treatment of choice. By contrast, the current evidence suggests that haploidentical donor transplantations, whether undertaken with or without conditioning, have a poor chance of success and in the conditioned setting are associated with a high mortality rate. We would therefore argue that haploidentical transplantations should be undertaken only if no other treatment options are available. At present, the data from matched unrelated donor transplantations (67% survival in only 11 patients in the European experience) do not provide conclusive evidence in favor of or against this approach because of the low numbers of patients treated and need to be set alongside data from other treatment options.
ERT
By maintaining high ADA activity in plasma, weekly or twice-weekly intramuscular injection of PEG-ADA eliminates Ado and dAdo derived from nucleotide and nucleic acid turnover. This protects immature lymphoid cells from apoptosis triggered by dAdo-induced dATP pool expansion, and from other mechanisms, restoring protective immune function in most patients within approximately 2 to 4 months.3 Systemic ERT may also prevent metabolic toxicity to other organs, which may cause hepatic and neurologic dysfunction in some ADA-deficient patients.
ERT requires appropriate biochemical (as well as immunologic) monitoring. Efficacy is associated with maintaining sufficient plasma ADA activity to eliminate dAdo nucleotides (dAXP) from erythrocytes. A decline in plasma ADA activity with reaccumulation of dAXP in red blood cells might indicate inadequate dosing, development of neutralizing anti-ADA antibody, improper storage of PEG-ADA (unstable if frozen), or nonadherence.
Unlike transplantation and GT, ERT can be performed by local physicians outside a transplantation center. However, with no central point for data collection, long-term follow-up of immunologic function in ERT patients has been difficult. However, the laboratory of M.S.H. has provided biochemical monitoring for most patients treated by ERT in the United States, and for many others in the 19 other countries in which PEG-ADA has been used since it was first tested in 1986. Updated records document demographics, length of ERT, and survival for approximately 185 patients treated with PEG-ADA through September 2008 (∼ 90% of those ever treated). (Enzon Pharmaceuticals and Orphan Europe have helped to verify current treatment status of patients who were no longer being monitored, but some uncertainty exists for a few patients.) Reviewing the available information may be useful in reaching consensus about the future role of ERT for ADA deficiency.
PEG-ADA has been used as initial therapy for patients who lacked a related HLA-identical marrow/stem cell donor, when assessment of risk and benefit by physicians and parents favored ERT over other options.22,25 Often, patients were considered too unstable to undergo conditioning for an immediate HLA-mismatched HSCT or to tolerate delay while searching for a matched unrelated donor or awaiting evaluation for a GT trial. Fewer than 10% of patients have received PEG-ADA as secondary therapy after failing HSCT (mainly haploidentical performed without conditioning) or GT.
Overall, 70% of patients treated with PEG-ADA had SCID and began ERT at younger than 1 year of age (50% were < 6 months old). Half of the remaining patients started treatment at 1 to 3 years of age, and half at 3 to 34 years of age. Many of these latter “delayed”- or “late”-onset patients had pulmonary disease or other consequences of chronic immune deficiency, which made them poor candidates for partially mismatched HSCT with conditioning.
As of September 2008, 98 patients were receiving PEG-ADA, approximately half of the number that had begun ERT (Figure 2). Approximately 20% of patients had died while on therapy; the remainder had discontinued ERT to undergo a potentially curative procedure (∼ 20% HSCT, ∼ 8% GT). More than two-thirds of the transplantations were performed within a year of starting PEG-ADA, as soon as the clinical condition was stable and a suitable donor had been identified. During the first decade after PEG-ADA received Food and Drug Administration approval in 1990, survival after these “elective” transplantations was approximately 50%,25 similar to that for partially mismatched transplantations in ADA-deficient SCID patients who had not received prior ERT (as summarized by others). In recent years, we have not been able to track outcome after HSCT.
Fate of patients who received ERT between April 1986 and September 2008.
Figure 3 plots the estimated probability of survival versus length of treatment with PEG-ADA. Half of the deaths on ERT occurred within the first 6 months (40% in the first month), resulting from conditions present at diagnosis. The overall probability of surviving 20 years on ERT is estimated to be 78%. A patient alive 6 months after starting ERT had approximately 90% probability of surviving the next 12 years. Conditions contributing significantly (3-5 patients each) to mortality beyond 6 months include refractory hemolytic anemia at 1 to 3 years, chronic pulmonary insufficiency after 5 to 15 years, and lymphoproliferative disorders after 5 to 15 years of ERT.26,27 Hepatocellular carcinoma developed in 2 patients, one just starting ERT after failing an unconditioned haploidentical HSCT and a second after 10 years of ERT. Another patient died of hepatoblastoma discovered after 2 years of ERT, but thought to be present at diagnosis of ADA deficiency. Late deaths resulting from acute infection appear to be uncommon, but a patient recently died of measles after 10 years of treatment.
Estimated probability of survival while receiving ERT. Patients who discontinued ERT to undergo stem cell transplantation or GT were censored at the time ERT was stopped, except for 5 patients who developed refractory hemolytic anemia and subsequently died after attempted stem cell transplantation. In these latter 5 cases, death was attributed to ERT at the time PEG-ADA was discontinued.
Estimated probability of survival while receiving ERT. Patients who discontinued ERT to undergo stem cell transplantation or GT were censored at the time ERT was stopped, except for 5 patients who developed refractory hemolytic anemia and subsequently died after attempted stem cell transplantation. In these latter 5 cases, death was attributed to ERT at the time PEG-ADA was discontinued.
We have identified neutralizing antibodies to PEG-ADA, which directly inhibit catalytic activity and accelerate clearance from plasma, in 9 patients.28,29 Seven of these patients either were able to continue ERT after dosage adjustment or underwent successful HSCT. However, loss of efficacy contributed to death in 4 patients, including 3 who also developed refractory hemolytic anemia.
Two-thirds of patients now receiving PEG-ADA have been treated for more than 5 years, including 20% for 15 to 22 years. In general, long-term ERT has been well tolerated, and most of these patients have remained free of opportunistic or abnormally frequent infections. Although our information is incomplete, earlier analysis indicated that half or more of these patients had discontinued intravenous immunoglobulin. One area of concern is the degree of immune reconstitution on long-term ERT. Serial studies of immune function have been reported in relatively few long-term patients, but in 2 recent reports a gradual decline in lymphocyte counts and various abnormalities in lymphocyte function, including thymic output, have been documented.30,31 These are in contrast to data available from HSCT recipients and a small number of patients treated by GT where long-term immune recovery both cellular and humoral appears to be maintained.
In summary, PEG-ADA provides an often life-saving therapy at the time of diagnosis, when other options may be unavailable or less predictably effective. If ERT is continued beyond 6 months, there is a high probability that clinical benefit can be sustained for at least a decade. However, PEG-ADA may not be easily available in some countries, and its high cost is a barrier to long-term ERT; and more uncertainty exists about how long-term immunologic and clinical benefit can be maintained beyond 8 to 10 years. An additional concern with ERT beyond about 8 to 10 years is the emergence of serious complications, including lymphoid and possibly hepatic malignancies, and progression of chronic pulmonary insufficiency.
GT for ADA-deficient SCID
As a severe monogenic disease, ADA deficiency is a compelling candidate for treatment with GT.32 Several clinical studies have investigated the safety and efficacy of ADA gene transfer into autologous hematopoietic cells using retroviral vectors. In initial trials conducted in the early 1990s, 19 patients received infusions of transduced lymphocytes33-35 or hematopoietic progenitor cells while continuing ERT.34,36 No toxicity was observed, and in most patients gene-modified T cells persisted in the circulation several years after infusion.37-39 However, the relatively low gene transfer efficiency and engraftment level of transduced cells observed in these patients did not lead to substantial immunologic improvement and clinical benefit.
A major progress was accomplished after the adoption of an improved gene transfer protocol into bone marrow CD34+ cells combined with the administration of a nonmyeloablative dose of busulfan chemotherapy before cell reinfusion.40 Since 2000, 15 children lacking an HLA-identical sibling donor were enrolled in this experimental protocol41 (and A.A., unpublished data, 2009). Most patients displayed an inadequate response to ERT or had failed mismatched related transplantation. To exploit the selective advantage for ADA-transduced cells in a toxic environment, no PEG-ADA was given after GT. The reduced dose of busulfan induced a transient myelosuppression without organ toxicity, which was sufficient to achieve long-term engraftment of gene-corrected hematopoietic stem cells.40 The dose of CD34+ cells infused and the efficiency of gene transfer were shown to be critical factors in determining a higher proportion of gene-corrected myeloid cells engrafting in patients. The large majority of lymphocytes were ADA-transduced, confirming that PEG-ADA withdrawal favors the selective survival of gene-corrected cells37,42 (and A.A., unpublished data, 2009). Clonal analysis of long-term repopulating cells revealed the presence of shared vector integrations among multiple hematopoietic lineages, thus proving the engraftment of multipotent hematopoietic stem cells.43 All children are alive, and the outcome of the first 10 treated patients has been recently reported. Eight of the 10 patients remain currently off ERT, with persisting ADA expression and systemic detoxification up to 8 years after treatment. Nine patients displayed recovery of polyclonal thymopoiesis, significant increase in T-cell counts (although 4 patients did not reach normal levels), and full correction of T-cell function, including susceptibility to apoptosis and proliferative responses to mitogens and antigens.44 Intravenous immunoglobulin treatment was discontinued in 5 children with evidence of antigen-specific antibodies to vaccinal antigens and pathogens. The progressive restoration of immune and metabolic functions led to significant improvement of patients' physical development and protection from severe infections, without adverse events related to GT.
In a similar trial performed in London using an alternative retroviral construct and a single-dose melphalan conditioning regimen, 5 patients have been treated45 (and H.B.G., unpublished data, 2009). Two patients have shown very good immune recovery and in a third, gene-transduced cells contribute to immune recovery, although PEG-ADA was restarted. Failure of GT was seen in 2 patients: in one case as a result of a poor stem cell harvest and in another because of low-level stem cell transduction efficiency. In the 2 most successfully reconstituted patients, ADA expression was observed in different hematopoietic lineages, including red blood cells, leading to effective metabolic detoxification. The large majority of T cells and NK cells were gene corrected, whereas significant gene marking was also observed in granulocytes and monocytes. In these 2 persons, reinitiation of thymopoiesis after GT was also noted.
The clinical trials conducted at the National Institutes of Health and Childrens Hospital of Los Angeles have provided important information on the role of myelosuppression in favoring engraftment of gene-corrected HSCs. In the first study, 4 patients undertook GT without receiving conditioning, resulting in low levels of marking in 2 patients and no sustained immunologic improvement (F.C., unpublished data, 2009). The second clinical trial included low-dose myelosuppression with busulfan and withdrawal of PEG-ADA and has enrolled 6 patients with a follow-up ranging between 2 months and 2.5 years. This version of the trial led to superior immunologic and metabolic outcome with 2 of 3 patients with a follow-up longer than 1 year having derived clinical benefit as shown by normalization of in vitro T function and improvement of immunoglobulin production that in one case allowed for normal responses to vaccinations. In all but one case, production of ADA by peripheral blood mononuclear cells is at levels between 30% to 100% of normal, leading to sustained metabolic control similar to that observed after HSCT (K. Shaw, personal communication, ASGCT 2009, San Diego, CA). One patient experienced a prolonged cytopenia after busulfan conditioning, as the consequence of a preexisting cytogenetic abnormality, pointing out at a potential limitation for patients subject to autologous gene transfer.46
Preliminary reports from a Japanese GT trial under which 2 ADA-SCID patients were also treated with retroviral gene transfer into CD34+ bone marrow cells after withdrawal of PEG-ADA treatment, but in the absence of any myeloablative conditioning, have suggested that some immunologic reconstitution can be observed in such a setting. Pending the availability of further details, the extent of the improvement in comparison with the results obtained in patients subjected to previous conditioning remains unclear.47
ADA deficiency represents a paradigmatic approach for GT approaches for inherited blood-borne disorders. The results of clinical trials indicate that GT with nonmyeloablative conditioning is associated with clinical benefit and is now an option to be considered for all ADA-SCID patients lacking an HLA-identical sibling donor. The results from the recent trials in Milan, London, and the United States in which at least 20 patients have been followed for longer than 1 year are impressive in terms of both toxicity and efficacy. All patients have survived therapy, which compares favorably with HSCT data, and the majority of patients have near full or partial recovery of immune function. With respect to nonimmunologic manifestations, it remains to be determined whether GT will be superior to allogeneic transplantation in preventing the neuro-cognitive complications frequently observed in these children after transplantation.23,48
Importantly, none of the patients showed clonal proliferation or adverse events related to gene transfer, despite observation of integration of vector sequences into known oncogenes. This may indicate that GT for ADA-SCID has a favorable risk-benefit profile, as opposed to GT for SCID-X1,49,50 although further long-term follow-up is necessary. Nevertheless, safety monitoring should be continued to be strictly implemented long term in all patients, according to guidelines of regulatory agencies. The development of self-inactivating vectors, such as lentiviral vectors,51,52 might further improve the safety of this approach.
Discussion and recommendations for management
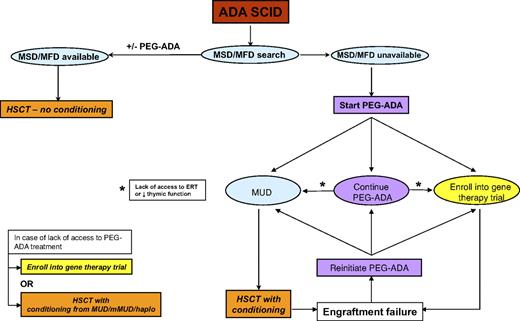
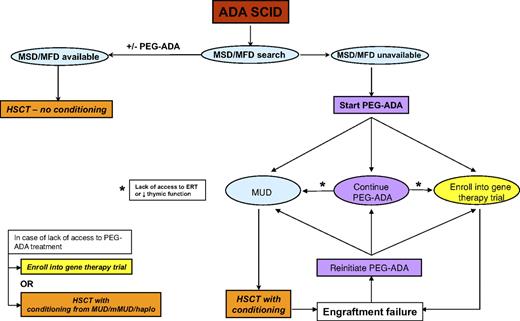
The evidence presented represents the current knowledge available for all 3 treatment modalities and now allows the authos to present a consensus “How we treat ADA deficiency” strategy. These are summarized in the flow diagram in Figure 4. We recognize that these opinions are based on currently available data, some of which have not been formally evaluated as part of peer-reviewed publications, and we also acknowledge that the opinions expressed may be subject to change as more patients are treated and data accrued.
Early diagnosis and stabilization and treatment of active infection are obviously of great importance as for any type of SCID and similarly so for ADA-SCID. We would also start PEG-ADA (if available) in any child who is clinically unwell, especially those with respiratory and nutritional difficulties. If a matched sibling or family donor (MSD or MFD) is available, then we would suggest that this should be the treatment of choice. The majority of MSD/MFD transplantations have been undertaken without any chemotherapy, and at present there is no robust argument for introducing conditioning, especially with the excellent survival data for unconditioned HSCT. The long-term outcome in unconditioned patients, certainly in terms of immune recovery, is good, and further evidence to the contrary would be necessary to suggest introduction of conditioning to promote better stem/progenitor cell engraftment. If PEG-ADA has been started, it is doubtful that there will have been significant immune recovery (which usually takes 3-6 months) before an MSD/MFD is found, in which case PEG-ADA can be stopped at the time of the unconditioned procedure.
If no MSD/MFD is available, we would start PEG-ADA to stabilize the child, achieve metabolic detoxification, and initiate immune recovery while a search for an unrelated or cord blood donor is undertaken. A clear message from the available data is that, in the absence of a matched unrelated or family donor, transplantation from a mismatched donor, and especially haploidentical donors, on which there is a convincing body of evidence, has a poor chance of success with or without conditioning. For these reasons, these mismatched transplantations should be undertaken only if there is no access to ERT, perhaps for economic reasons, or if there is no possibility of enrolling the child into a trial of GT. Cord blood donations may tolerate a greater degree of HLA disparity than adult unrelated donors, although there is presently insufficient evidence in the ADA SCID HSCT database to inform any strong recommendation.
The most difficult decisions arise when faced with the 3 possibilities of continuing with ERT, opting for a conditioned matched unrelated donor (MUD) HSCT, or treating the child by GT. Once started on PEG-ADA and if the child survives beyond the first 6 months, there is a very high likelihood of clinical well-being and effective immune recovery over at least a 5-year period. One option would be to continue indefinitely on PEG-ADA, and this is dependent on the long-term availability of access to ERT and of the parental and physician attitude to the risks associated with HSCT or GT. One important consideration is the long-term immunologic recovery on ERT, which we know from long-term follow-up of patients is suboptimal with low T-cell numbers and decreased thymic function. Not only may this predispose to the development of infective or lymphoproliferative sequelae, but deterioration in thymic function over time may preclude the chance of successful treatment by either HSCT or GT at a later stage. For these reasons, we would monitor immune and thymic function (by TREC or naive T-cell phenotype enumeration) on a regular 6-month basis for any child on long-term ERT, with a view to offering definitive therapy if deterioration of naive T-cell output is seen. A further reason to discontinue ERT and offer HSCT or GT would be the development of autoimmune cytopenias or neutralizing antibody that was refractory to immune modulation.
In the event of poor immune recovery on ERT or the lack of access to long-term ERT, then definitive therapy by HSCT or GT should be considered. Some physicians and parents would also want to undertake HSCT or GT once a donor has been found and once the child has been stabilized rather than continuing on long-term ERT. This would be a personal choice, and at present it is not possible to make a strong recommendation on long-term ERT versus short-term stabilizing ERT followed by HSCT/GT and is a choice dependent on the immune recovery on ERT, access to ERT, and personal attitude to risk.
If a decision has been made to undertake a definitive procedure, then it is necessary to consider the merits of an unrelated donor transplantation and GT. HSCT is a procedure that is readily undertaken by a large number of centers worldwide and is readily available, even if the experience with ADA SCID may be limited. The results of fully matched UD transplantations are limited, with a survival of 67% in only 11 patients treated. These outcome values could easily improve or worsen depending on the results from a handful of further transplantations, and it is therefore difficult to draw significant conclusions. It is probable, however, that the greatest risk to patients will occur within the first few months after transplantation; and if patients survive the first year, then there is the likelihood of long-term event-free survival with excellent cellular and humoral immune recovery. Our own clinical experience suggests that ADA-SCID patients do not tolerate chemotherapy or stress resulting from infection as well as other SICD patients, possibly because of the metabolic nature of the disease. Thus, patients should be clear of active infection and in good nutritional state before transplantation. Conditioning before a transplantation is advisable because, as previously stated, 2 of 4 MUD transplantations performed without conditioning did not engraft. The choice of conditioning regimen is important, but at present there is no consensus on the best conditioning regimen to use in this disease. Here again, our experience, rather than evidence, would support a reduced-intensity conditioning regimen, and we would advise continuing ERT up until the start of chemotherapy.
Compared with HSCT, GT has the advantage of being potentially applicable to all patients, without delay and independently from the availability of an allogeneic donor. The use of autologous gene-corrected cells eliminates the chance of rejection and the risks of complications resulting from graft-versus-host disease, immunosuppressive prophylaxis, and higher-dose conditioning regimens. Unlike ERT, a single infusion of transduced HSCs should be sufficient to treat a patient life-long, reducing the patient's burden in terms of costs and quality of life. Moreover, several lines of evidence suggest that endogenous cellular ADA is more effective than exogenously administered ADA53 in recovery of T lymphocyte function and long-term thymic recovery.30 The lower-intensity conditioning regimens used for GT in ADA-SCID and the use of autologous cells, thereby avoiding graft-versus-host disease, are probably the main factors for the excellent survival figures associated with GT, and to date all patients have survived. On the other hand, GT carries an inherent risk of insertional mutagenesis associated with retroviral insertions at sensitive genomic sites,54 which currently remains low based on the absence of such complications in at least 35 patients treated since 1991, including 17 of 24 patients treated since 2000 who have durable immune recovery and are off ERT. Thus, the choice between HSCT and GT may be summarized as a choice between short-term and potential long-term toxicities. HSCT is associated with toxicities in the year after treatment, after which long-term survival and effective immune recovery are highly probable. GT poses minimal toxicity within the first year and a good chance of immune recovery but retains the potential for long-term toxicity using current vector technology. The balance between the 2 modalities will need to be reviewed on a regular basis as data emerge from more unconditioned transplantations using less toxic conditioning regimens and long-term follow-up of GT patients and the anticipated use of alternative vectors with decreased potential for insertional mutagenesis.
Further issues surround the best treatment for the nonimmunologic manifestations of ADA-SCID and, most importantly, the cognitive and neurologic defects. Currently, data are available from only those patients who have undergone HSCT, which highlights the incidence of mild to severe problems in this cohort. No data are available from patients undergoing ERT or GT. It may be hypothesized that better systemic detoxification may result in an amelioration of nonimmunologic abnormalities, and it will be important to formally study these patients and plan the analysis. At present, however, these considerations cannot guide any specific treatment choice.
ADA-SCID remains a challenging condition to treat. The availability of different management options without formal outcome studies has made the choice of treatment difficult. Emerging data reviewed in this report provide the basis to make clear recommendations in some specific areas. However, as outlined, the choice among ongoing ERT, MUD HSCT, and GT remains difficult because of the absence of large-scale outcome studies and the different risks/benefits associated with the 3 treatment options. We have attempted to outline the balance between the different options and suggest specific triggers, which may indicate when patients should move from ERT to HSCT or GT. As further data are accrued, the choices may become easier to make; but until then, decision-making will still be dependent not only on certain practicalissues such as financial access to long-term ERT or availability of GT but also on the parental and physician attitude to the short-term and potential long-term risks associated with the different treatment options.
Acknowledgments
The authors would like to acknowledge all physicians who have contributed transplantation and patient data.
F.C. was supported in part by funding from the National Human Genome Research Institute/National Institutes of Health intramural research program. A.A. has received grant support from the Fondazione Telethon. H.B.G is supported in part from the Great Ormond Street Hospital Biomedical Research Center.
National Institutes of Health
Authorship
Contribution: All authors discussed the format and content of the article and contributed to the writing, review, and editing of the final manuscript.
Conflict-of-interest disclosure: M.S.H. has received research support from Enzon Pharmaceuticals Inc and is an occasional consultant to Enzon and Orphan Europe. H.B.G. is an occasional consultant to Enzon. L.D.N., A.A., F.P., and F.C. declare no competing financial interests.
Correspondence: H. Bobby Gaspar, Molecular Immunology Unit, UCL Institute of Child Health, 30 Guilford St, London WC1N 1EH, United Kingdom; e-mail: h.gaspar@ich.ucl.ac.uk.