Abstract
The administration of cytokines that modulate endogenous or transferred T-cell immunity could improve current approaches to clinical immunotherapy. Interleukin-2 (IL-2) is used most commonly for this purpose, but causes systemic toxicity and preferentially drives the expansion of CD4+CD25+Foxp3+ regulatory T cells, which can inhibit antitumor immunity. IL-15 belongs to the γc cytokine family and possesses similar properties to IL-2, including the ability to induce T-cell proliferation. Whereas IL-2 promotes apoptosis and limits the survival of CD8+ memory T cells, IL-15 is required for the establishment and maintenance of CD8+ T-cell memory. However, limited data are available to guide the clinical use of IL-15. Here, we demonstrate in nonhuman primates that IL-15 administration expands memory CD8+ and CD4+ T cells, and natural killer (NK) cells in the peripheral blood, with minimal increases in CD4+CD25+Foxp3+ regulatory T cells. Daily administration of IL-15 resulted in persistently elevated plasma IL-15 levels and transient toxicity. Intermittent administration of IL-15 allowed clearance of IL-15 between doses and was safe for more than 3 weeks. These findings demonstrate that IL-15 has profound immunomodulatory properties distinct from those described for IL-2, and suggest that intermittent administration of IL-15 should be considered in clinical studies.
Introduction
The administration of high doses of interleukin-2 (IL-2) is effective in promoting tumor regression in a small subset of patients with renal cell carcinoma and melanoma,1-3 and IL-2 is frequently administered after the adoptive transfer of tumor or virus-specific T cells to support their in vivo survival.4,5 However, IL-2 can cause substantial systemic toxicity, particularly when administered in high daily doses,6 and promotes the expansion of regulatory CD4+ T cells, which inhibit antitumor immunity.7 Thus, alternative cytokines that can be administered without toxicity to modulate endogenous or transferred T-cell immunity may be beneficial.
IL-15, like IL-2, belongs to the 4 α-helix bundle family of cytokines and shares some functional activities with IL-2, including binding to the IL-2 β and γc receptor (R) chains, and promoting the proliferation of activated T cells in vitro.8-10 IL-15 and IL-2 differ in that they bind to private IL-15Rα and IL-2Rα chains,11,12 and IL-15 has functions that are distinct from IL-2. IL-2Rα is largely restricted in its expression to activated and regulatory T cells and exhibits low affinity for IL-2 in the absence of the IL-2Rβγc, whereas IL-15Rα is expressed by activated monocytes, dendritic cells, a variety of tissue cells, and T cells.12 IL-15 binds IL-15Rα with high affinity, and can subsequently provide signals to T cells in cis through binding IL-15Rα IL-2Rβγc complexes; or in trans by interactions between IL-15Rα–bearing cells and neighboring IL-2Rβγc–bearing T cells.13,14 Moreover, endosomal recycling of IL-15Rα with its bound active ligand may provide for prolonged signaling.13 Gene targeting of IL-15, IL-2, and their private receptor components has revealed distinct roles for these cytokines in vivo. Mice rendered deficient in IL-15 or IL-15Rα have a marked reduction in peripheral natural killer (NK) cells, NKT cells, and CD8+ memory T cells,15-18 whereas mice deficient in IL-2 or IL-2Rα develop lymphoid hyperplasia and autoimmunity.11,19,20
Because of its critical and nonredundant role in establishing and maintaining T-cell memory, IL-15 is a potential alternative to IL-2 for augmenting endogenous or adoptively transferred T-cell immunity in humans.21 IL-15 has been shown to accelerate immune reconstitution after bone marrow (BM) transplantation in mice,22 and overexpression of IL-15 or administration of IL-15 alone or complexed with IL-15Rα, protects mice from some infections, enhances vaccination including in settings of CD4+ T-cell deficiency, and promotes destruction of established experimental tumors.23-28 Furthermore, the administration of IL-15 in conjunction with chemotherapy, Toll-like receptor agonists, or adoptive transfer of tumor-reactive CD8+ T cells can increase survival or tumor regression in murine models compared with each therapy alone.29-32
There are limited data on the safety and biologic effects of IL-15 in primates to guide its use in the clinic. Many of the studies in mice used human IL-15, which is active on murine T cells, but binds to the murine IL-2Rβγc with lower affinity than murine IL-15 and is only 73% identical in amino acid sequence.33 By contrast, human and macaque IL-15 are 97% identical in amino acid sequence and exhibit similar binding to IL-15 receptors, suggesting that studies of human IL-15 in nonhuman primates will likely be more predictive of activity and toxicity in humans.33 A small number of studies have administered simian IL-15 produced as a recombinant molecule in bacteria to normal rhesus macaques in an effort to improve T-cell responses to vaccination, or to simian immunodeficiency virus (SIV)–infected animals in an attempt to restore T-cell numbers.34-36 The half life (t1/2) of IL-15 after a single subcutaneous dose in normal animals was reported to be 0.92 to 1.31 hours, and administration of 10 μg/kg IL-15 every 2 to 7 days increased memory T-cell responses to tetanus toxoid and influenza virus vaccines.34 In SIV-infected macaques that concurrently received antiretroviral therapy, IL-15 given in doses of 10 to 100 μg/kg twice a week for up to 4 weeks boosted the numbers of circulating NK cells, CD8+ T effector memory (TEM) cells, and CD4+ TEM cells, and promoted the migration of CD4+ TEM cells into extralymphoid tissues.35,36 These studies did not report toxicity from administering IL-15, even at the highest doses, but the short t1/2 of IL-15 might suggest the need for more frequent dosing for some therapeutic applications.
We administered recombinant IL-15 produced in mammalian cells to retain glycosylation subcutaneously in a daily or intermittent schedule to normal macaques and evaluated toxicity and immunologic effects. IL-15 induced the proliferation of CD8+ and CD4+ T cells and NK cells, but repeated daily dosing resulted in persistently elevated plasma levels of IL-15, and was associated with transient toxicity. By contrast, the intermittent administration of IL-15 was well tolerated, and increased proliferation of CD8+ memory T cells, with only a minor increase in the absolute numbers of CD4+CD25+Foxp3+ regulatory T cells. These results, which are the first in nonhuman primates using recombinant IL-15 produced in mammalian cells, demonstrate the potential for toxicity from daily administration and suggest that an intermittent dosing schedule should be considered in clinical studies of IL-15 for immunomodulation.
Methods
Animals and toxicity monitoring
Adult macaques (Macaca nemestrina) were housed at the University of Washington National Primate Research Center (WaNPRC), under American Association for Accreditation of Laboratory Animal Care approved conditions. Protocols were approved by the Institutional Review Board and Animal Care and Use Committee. Human recombinant IL-15 produced in Chinese hamster ovary cells was kindly provided by Amgen,33 and was given in doses of 2.5 to 15 μg/kg by subcutaneous injection. Animals were monitored for clinical toxicity, and complete blood count and serum chemistry were measured in accredited clinical laboratories. A BM aspirate and biopsy were obtained from the femur. The biopsy was fixed in formalin and embedded in paraffin, and 6-micrometer sections were stained with hematoxylin and eosin.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were analyzed by cell-surface staining with the following fluorochrome-conjugated monoclonal antibodies (mAbs): CD3, CD4, CD8, CD16, CD20, CD28, CD56, CD62L, CCR7, CD95, isotype-matched irrelevant control mAbs (BD Biosciences), and anti–human TCRγδ (clone B1.1; eBioscience). Analysis was performed on a FACSCalibur flow cytometer and data were analyzed using CellQuest Software (BD Biosciences). Polychromatic flow cytometric analysis was performed on a 3-laser BD LSR II instrument using Pacific Blue, AmCyan, fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-Cy7, allophycocyanin, peridinin chlorophyll protein-Cy5.5, and allophycocyanin-Cy7 as fluorescent parameters.36 Data were analyzed using FlowJo software (Version 6.3.1; TreeStar Inc). To assess intracellular protein expression, cells were permeabilized using fluorescence-activated cell sorting (FACS) Permeabilizing Solution, and then stained with a FITC-labeled mAb that binds to Ki-67 (BD Biosciences). To enumerate regulatory CD4+ T cells, PBMCs were stained with mAbs reactive to CD4 (peridinin chlorophyll protein-Cy5.5), CD3 (FITC), and CD25 (PE), and then lysed and permeabilized using the Foxp3 Buffer Set (BD Biosciences), and stained for intracellular Foxp3 (BD). In some experiments, PBMCs were stained with annexin V and propidium iodide according to the manufacturer's instructions (BD Biosciences).
Cytokine flow cytometry assay for detection of CMV-specific memory T cells
Cytokine flow cytometry (CFC) was used to detect CD8+ T cells in PBMCs that expressed intracellular interferon-gamma (IFN-γ) after stimulation with rhesus cytomegalovirus (CMV) immediate early 1 or pp65 peptides as described.37 Briefly, aliquots of PBMCs obtained before, during, and after IL-15 administration were thawed on the same day and stimulated for 6 hours at 37°C with peptide (5 μg/mL) or medium alone in the presence of anti-CD28 and anti-CD49d mAbs (1 μg/mL; BD Biosciences). After 2 hours, brefeldin A (10 μg/mL; Sigma-Aldrich) was added. Samples were stained for cell-surface expression of CD8 and CD3, permeabilized using FACS Permeabilizing Solution, stained with an IFN-γ mAb (BD Biosciences), and analyzed as described.37 In some experiments, permeabilized cells were simultaneously stained with a PE-labeled Ki-67 mAb.
IL-15 ELISA
Aliquots of plasma obtained before or after the IL-15 injection were analyzed for the presence of IL-15 using an enzyme-linked immunosorbent assay (ELISA) kit that detects human and macaque IL-15 (R&D Systems) according to the manufacturer's instruction. The detection level of the assay is 0.3 to 1.2 pg/mL.
Evaluation of T-cell receptor Vβ gene use
T-cell receptor (TCR) Vβ gene use was assessed using a reverse transcriptase–polymerase chain reaction (RT-PCR) method based upon measurement of the complementary determining region 3 length.38 Briefly, total RNA was extracted from peripheral blood T-cell subsets (RNeasy Mini Kit; QIAGEN). Complementary DNA was synthesized from RNA using a Superscript III cDNA synthesis kit (Invitrogen). The TCR Vβ gene use was determined by RT-PCR using macaque-specific TCR Vβ primers and a single TCR Cβ primer as described.38
Statistical analysis
A statistical analysis was performed to examine whether changes in absolute or relative numbers of different cell populations during the IL-15 treatment were different from baseline. Briefly, the difference from baseline for weeks 1, 2, and 3, respectively, was calculated for each of the animals. We next determined the average of these 3 values for each animal and used a 1-sample t test to examine whether these values were different from zero. To examine the increase in the proportion of Ki-67+ cells, we compared week 1 and the pretreatment value.
Results
Toxicity of daily subcutaneous IL-15 administration in macaques
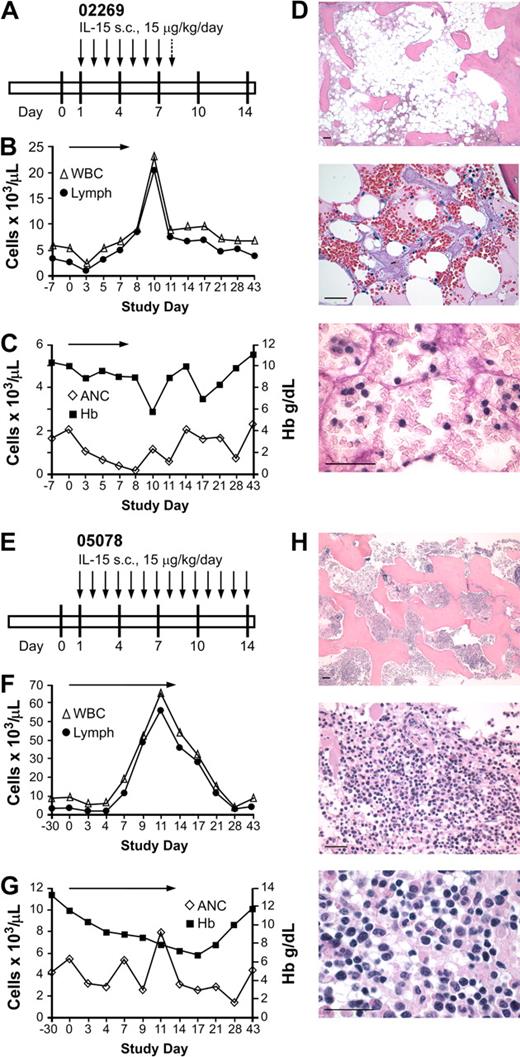
There is considerable experience administering IL-2 to animals and humans,1-7 but much less information on the toxicity and immunologic effects of IL-15. Most cytokines, including IL-15, are present in very low or undetectable levels in normal serum, suggesting that tight regulation is key to their normal biologic functions and to prevent toxicity.21 Based on the short t1/2 of IL-15 after subcutaneous injection in primates reported previously,34 we speculated that daily administration might be required to achieve immunomodulatory effects. In a pilot study, we evaluated the effects of IL-15 given daily for up to 14 days and monitored clinical toxicity, complete blood count, and differential, serum chemistry, and peak and trough IL-15 levels. Two macaques received IL-15 at a daily dose of 15 μg/kg per day (Figure 1A,E). In macaque 02269, the white blood cell count (WBC) increased from 2.59 × 109/L (2590/μL) before treatment to 8.36 × 109/L (8360/μL) by day 7, due to an increase in lymphocyte numbers (Figure 1B). However, the absolute neutrophil count (ANC) began to decline on day 3 and was less than .5 × 109/L (500/μL) by day 7 of treatment (Figure 1C). The animal did not exhibit any clinical toxicity or abnormal serum chemistry, and the platelet count and hematocrit remained stable. A BM biopsy obtained on day 7 showed hypocellularity (Figure 1D), consistent with lack of neutrophil production rather than accelerated turnover or migration from the blood pool. A further decline in the ANC to .09 × 109/L (90/μL) by day 8 prompted the discontinuation of IL-15, and the ANC recovered to normal levels over the next 7 days (Figure 1C). The total WBC and lymphocyte counts continued to increase after discontinuation of IL-15, peaking on day 10 at 23.1 × 109/L (23 100/μL) and 20.33 × 109/L (20 330/μL), respectively, before declining to near baseline levels by day 28 (Figure 1B).
Effect of daily subcutaneous IL-15 in 2 immunocompetent macaques. (A) Schedule of IL-15 administration (15 μg/kg per day). Each  represents a single daily dose of IL-15 (15 μg/kg), and the
represents a single daily dose of IL-15 (15 μg/kg), and the  indicates a single daily dose of IL-15 (5 μg/kg). (B-C) Absolute numbers of WBCs, lymphocytes, and neutrophils (ANCs) in the peripheral blood before, during, and after IL-15 therapy. Absolute cell numbers of (B) WBCs (▵) and lymphocytes (●) per microliter of peripheral blood or (C) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) were determined on the indicated days. The arrows indicate the duration of the IL-15 administration. (D) Photomicrographs of hematoxylin and eosin–stained BM biopsy sections obtained after 7 days of treatment display hypoplasia and moderate focal hemorrhage (top, 5× objective). At higher magnification (middle, 40× objective), there is also fat necrosis and stromal edema. The scattered cells are erythroid precursors, lymphocytes, and macrophages. Normal clusters of maturing myeloid cells are absent (bottom, 100× objective). Photomicrographs were obtained with a Leica DFC320 camera on a Leica DM3000 microscope and processed with the Leica Application Suite version 3.1.0 (Leica Microsystems GmbH). Images were processed using Photoshop 7.0 software (Adobe Systems). Bars represent 100 micrometers. (E) Schedule of IL-15 administration for macaque 05078 (15 μg/kg per day). Each
indicates a single daily dose of IL-15 (5 μg/kg). (B-C) Absolute numbers of WBCs, lymphocytes, and neutrophils (ANCs) in the peripheral blood before, during, and after IL-15 therapy. Absolute cell numbers of (B) WBCs (▵) and lymphocytes (●) per microliter of peripheral blood or (C) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) were determined on the indicated days. The arrows indicate the duration of the IL-15 administration. (D) Photomicrographs of hematoxylin and eosin–stained BM biopsy sections obtained after 7 days of treatment display hypoplasia and moderate focal hemorrhage (top, 5× objective). At higher magnification (middle, 40× objective), there is also fat necrosis and stromal edema. The scattered cells are erythroid precursors, lymphocytes, and macrophages. Normal clusters of maturing myeloid cells are absent (bottom, 100× objective). Photomicrographs were obtained with a Leica DFC320 camera on a Leica DM3000 microscope and processed with the Leica Application Suite version 3.1.0 (Leica Microsystems GmbH). Images were processed using Photoshop 7.0 software (Adobe Systems). Bars represent 100 micrometers. (E) Schedule of IL-15 administration for macaque 05078 (15 μg/kg per day). Each  represents a single daily dose of IL-15. (F) Absolute cell numbers of WBCs (▵) and lymphocytes (●) per microliter of peripheral blood before, during, and after IL-15 therapy. (G) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) before, during, and after IL-15 therapy.
represents a single daily dose of IL-15. (F) Absolute cell numbers of WBCs (▵) and lymphocytes (●) per microliter of peripheral blood before, during, and after IL-15 therapy. (G) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) before, during, and after IL-15 therapy.  indicates the duration of the IL-15 administration. (H) Photomicrographs of hematoxylin and eosin–stained marrow biopsy sections obtained after the IL-15 treatment as described in panel D. Bars represent 100 micrometers.
indicates the duration of the IL-15 administration. (H) Photomicrographs of hematoxylin and eosin–stained marrow biopsy sections obtained after the IL-15 treatment as described in panel D. Bars represent 100 micrometers.
Effect of daily subcutaneous IL-15 in 2 immunocompetent macaques. (A) Schedule of IL-15 administration (15 μg/kg per day). Each  represents a single daily dose of IL-15 (15 μg/kg), and the
represents a single daily dose of IL-15 (15 μg/kg), and the  indicates a single daily dose of IL-15 (5 μg/kg). (B-C) Absolute numbers of WBCs, lymphocytes, and neutrophils (ANCs) in the peripheral blood before, during, and after IL-15 therapy. Absolute cell numbers of (B) WBCs (▵) and lymphocytes (●) per microliter of peripheral blood or (C) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) were determined on the indicated days. The arrows indicate the duration of the IL-15 administration. (D) Photomicrographs of hematoxylin and eosin–stained BM biopsy sections obtained after 7 days of treatment display hypoplasia and moderate focal hemorrhage (top, 5× objective). At higher magnification (middle, 40× objective), there is also fat necrosis and stromal edema. The scattered cells are erythroid precursors, lymphocytes, and macrophages. Normal clusters of maturing myeloid cells are absent (bottom, 100× objective). Photomicrographs were obtained with a Leica DFC320 camera on a Leica DM3000 microscope and processed with the Leica Application Suite version 3.1.0 (Leica Microsystems GmbH). Images were processed using Photoshop 7.0 software (Adobe Systems). Bars represent 100 micrometers. (E) Schedule of IL-15 administration for macaque 05078 (15 μg/kg per day). Each
indicates a single daily dose of IL-15 (5 μg/kg). (B-C) Absolute numbers of WBCs, lymphocytes, and neutrophils (ANCs) in the peripheral blood before, during, and after IL-15 therapy. Absolute cell numbers of (B) WBCs (▵) and lymphocytes (●) per microliter of peripheral blood or (C) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) were determined on the indicated days. The arrows indicate the duration of the IL-15 administration. (D) Photomicrographs of hematoxylin and eosin–stained BM biopsy sections obtained after 7 days of treatment display hypoplasia and moderate focal hemorrhage (top, 5× objective). At higher magnification (middle, 40× objective), there is also fat necrosis and stromal edema. The scattered cells are erythroid precursors, lymphocytes, and macrophages. Normal clusters of maturing myeloid cells are absent (bottom, 100× objective). Photomicrographs were obtained with a Leica DFC320 camera on a Leica DM3000 microscope and processed with the Leica Application Suite version 3.1.0 (Leica Microsystems GmbH). Images were processed using Photoshop 7.0 software (Adobe Systems). Bars represent 100 micrometers. (E) Schedule of IL-15 administration for macaque 05078 (15 μg/kg per day). Each  represents a single daily dose of IL-15. (F) Absolute cell numbers of WBCs (▵) and lymphocytes (●) per microliter of peripheral blood before, during, and after IL-15 therapy. (G) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) before, during, and after IL-15 therapy.
represents a single daily dose of IL-15. (F) Absolute cell numbers of WBCs (▵) and lymphocytes (●) per microliter of peripheral blood before, during, and after IL-15 therapy. (G) ANCs (◇) per microliter of peripheral blood and level of hemoglobin (■ Hb; gd/L) before, during, and after IL-15 therapy.  indicates the duration of the IL-15 administration. (H) Photomicrographs of hematoxylin and eosin–stained marrow biopsy sections obtained after the IL-15 treatment as described in panel D. Bars represent 100 micrometers.
indicates the duration of the IL-15 administration. (H) Photomicrographs of hematoxylin and eosin–stained marrow biopsy sections obtained after the IL-15 treatment as described in panel D. Bars represent 100 micrometers.
The second macaque, 05078, received IL-15 for the planned 14 days (Figure 1E). In this macaque, the pretreatment WBC and lymphocyte counts were 9.1 × 109/L (9100/μL) and 2.8 × 109/L (2810/μL), respectively, and increased to 65.5 × 109/L (65 500/μL) and 55.7 × 109/L (55 700/μL), respectively, by day 11 (Figure 1F). The ANC remained in the normal range in this animal, but the hemoglobin declined (Figure 1G) and the animal experienced a weight loss of more than 20% during the treatment regimen. In contrast to the first animal where the BM obtained on day 8 was hypocellular, the BM obtained from 05078 showed increased cellularity due to a marked infiltration with lymphocytes (Figure 1H).
Due to the hematopoietic and clinical toxicity observed in macaques 02269 and 05078 with administration of IL-15 at 15 μg/kg per day, we treated a third macaque (02050) with a lower daily dose of IL-15 (5 μg/kg). On the third day of IL-15, this animal developed a maculopapular skin rash that became generalized and was associated with a low-grade fever (100.8°F), pruritus, periorbital edema, and exfoliation despite treatment with diphenhydramine. There were no alterations in serum chemistry or other clinical parameters. A skin biopsy taken on day 5 of IL-15 treatment showed a mild, nonspecific dermatitis with scattered small lymphocytes infiltrating the epidermis and in a perivascular distribution in the dermis. IL-15 was discontinued on day 5, and the skin rash then resolved over 11 days. With this short course of low-dose IL-15, the ANC, WBC, and lymphocyte counts remained within normal limits (data not shown).
Daily IL-15 administration results in persistent elevation of IL-15 in plasma
IL-15 transcripts are expressed in many tissues, but plasma levels are low or undetectable due to transcriptional and translational regulation, and binding of IL-15 to cells that express IL-15Rα.12,39-43 We measured plasma IL-15 in macaques that received daily IL-15 before therapy, 2 hours after the third IL-15 injection to determine a peak level, and 24 hours after the second injection to determine a trough level (Table 1). IL-15 was undetectable or very low before treatment, but unexpectedly significant levels of IL-15 were detected in plasma obtained before the third injection in all macaques (Table 1). These trough levels were not consistent with the short half-life measured after a single injection of IL-15 produced in bacteria in a prior study,34 and could reflect differences in the IL-15 preparations or result from repeated daily injection of IL-15.
Immunologic effects of daily IL-15 administration
The composition of mononuclear cells in PBMCs obtained before and during daily IL-15 was examined by flow cytometry. In macaque 02269, the increase in the absolute lymphocyte count was due primarily to increased absolute numbers of CD3+CD8+ T cells, CD16+ NK cells, and CD4+ T cells (Figure 2A). In macaque 05078, the absolute numbers of CD3+CD8+ T cells, CD16+ NK cells, and CD4+ T cells also increased during the IL-15 treatment (Figure 2B). Analysis of the TCR repertoire of the peripheral blood T cells of the animals showed the expansion remained polyclonal (data not shown). However, in macaque 05078, the most dramatic increase in cell number occurred in CD3+CD4−CD8− T cells that were TCRγδ+ (Figure 2C-D). These cells rose from 233/μL before therapy to 46 955/μL on day 11 of IL-15 administration (Figure 2C-D), and then slowly declined to 329/μL by 6 weeks after the treatment (Figure 2D). Analysis of the lymphocyte infiltrate in the BM aspirate obtained from this animal (05078) demonstrated the infiltrate was predominantly CD3+CD4−CD8− TCRγδ+ T cells (data not shown). In macaque 02269, the subset of CD3+CD4−CD8− T cells increased from 52 cells/μL before therapy to 966 cells/μL by day 10 of the IL-15 treatment (data not shown).
Analysis of lymphocyte subsets after daily administration of IL-15. (A-B) Samples of PBMCs were obtained from macaque 02269 (A) or macaque 05078 (B) before, during, and after the IL-15 treatment. Aliquots of PBMCs were stained with mAbs binding to CD3, CD4, CD8, or CD16, and examined by flow cytometry. The data for CD3+CD4+ (▵), CD3+CD8+ (●), and CD16+ (♦) are shown as the absolute cell number of each subset per microliter of blood at the indicated days. (C) Representative sample of PBMCs obtained from macaque 05078 at the end of the IL-15 treatment. Aliquots of PBMCs were stained with mAbs binding to CD3, CD4, CD8, and a TCRγδ-specific mAb, and examined by flow cytometry. (Left panel) Cells are gated on CD3+ T cells. The values indicate the proportion of each of the T-cell subsets (in percentage). (Right panel) TCRγδ T cells within the PBMC population. (D) The data for CD3+CD4−CD8− cells (◇) are shown as the absolute cell number of each subset per microliter of blood at the indicated days. (E-F) Expression of Ki-67. Aliquots of PBMCs from macaque 02269 (E) or macaque 05078 (F) were obtained before, during, or after the IL-15 treatment and were examined for intracellular expression of Ki-67 within the CD3+CD4+, CD3+CD8+, or CD3+CD4−CD8− T-cell compartment, and within the CD16+ NK cell subset. The fraction of Ki-67–expressing cells in percentage (%) in each of the subsets on the indicated days before, during, and after the IL-15 treatment is shown.  indicates the duration of the IL-15 administration.
indicates the duration of the IL-15 administration.
Analysis of lymphocyte subsets after daily administration of IL-15. (A-B) Samples of PBMCs were obtained from macaque 02269 (A) or macaque 05078 (B) before, during, and after the IL-15 treatment. Aliquots of PBMCs were stained with mAbs binding to CD3, CD4, CD8, or CD16, and examined by flow cytometry. The data for CD3+CD4+ (▵), CD3+CD8+ (●), and CD16+ (♦) are shown as the absolute cell number of each subset per microliter of blood at the indicated days. (C) Representative sample of PBMCs obtained from macaque 05078 at the end of the IL-15 treatment. Aliquots of PBMCs were stained with mAbs binding to CD3, CD4, CD8, and a TCRγδ-specific mAb, and examined by flow cytometry. (Left panel) Cells are gated on CD3+ T cells. The values indicate the proportion of each of the T-cell subsets (in percentage). (Right panel) TCRγδ T cells within the PBMC population. (D) The data for CD3+CD4−CD8− cells (◇) are shown as the absolute cell number of each subset per microliter of blood at the indicated days. (E-F) Expression of Ki-67. Aliquots of PBMCs from macaque 02269 (E) or macaque 05078 (F) were obtained before, during, or after the IL-15 treatment and were examined for intracellular expression of Ki-67 within the CD3+CD4+, CD3+CD8+, or CD3+CD4−CD8− T-cell compartment, and within the CD16+ NK cell subset. The fraction of Ki-67–expressing cells in percentage (%) in each of the subsets on the indicated days before, during, and after the IL-15 treatment is shown.  indicates the duration of the IL-15 administration.
indicates the duration of the IL-15 administration.
To assess whether the increase in NK cells, and CD4+ and CD8+ T cells in 02269 and 05078 was due to cell proliferation, PBMCs were stained for Ki-67, a nuclear antigen expressed by cells undergoing proliferation. At baseline, less than 10% of CD4+ and CD8+ T cells were Ki-67+, but by day 7 after initiating IL-15, 37.4% to 60.3% of CD4+ T cells and 62.4% to 80.3% of CD8+ T cells were Ki-67+ (Figure 2E-F). The proportion of CD16+ NK cells that expressed Ki-67 was also increased (Figure 2E-F). A lesser, but significant, increase in Ki-67+ T cells and NK cells was observed with IL-15 at 5 μg/kg per day (data not shown). In both animals, 88% to 96% of the CD3+CD4−CD8− T cells were Ki-67+ by day 7 of IL-15 treatment, and the Ki-67+ fraction remained elevated for several days after IL-15 was discontinued.
Despite the large fraction of CD4+ T cells that were Ki-67+, the absolute numbers of CD4+ T cells in the peripheral blood increased to a lesser extent than CD8+ T cells and NK cells. This could be due to differential migration from the blood to lymph nodes or tissue sites, and a prior study demonstrated that IL-15 can promote the migration of CD4+ T cells to tissue sites.36 An alternative explanation is that CD4+ T cells induced by IL-15 to divide undergo increased cell death in vivo. We stained samples of PBMCs obtained from macaque 02269 and 05078 for annexin V and propidium iodide that might detect dying or dead cells and did not observe increased cell death in the CD4+ T-cell subset, although this was performed on cryopreserved cells (data not shown).
The CD4 and CD8 subsets contain naive (TN), central memory (TCM), and TEM populations that might respond differently to IL-15. TN and TCM cells are both CCR7+CD62L+CD28+ but can be distinguished from each other by differential expression of Fas, whereas CD62L− TEM cells can be identified as CD95+, CCR7−, and CD28−/low.36 We used CD95 and CCR7 to distinguish TN (CD95−/lowCCR7+), TCM (CD95+CCR7+), and TEM (CD95+CCR7−) CD8+ and CD4+ T-cell subsets (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article), and determined the absolute and relative number of each subset before and during the administration of IL-15. There was a 6.8- to 13.4-fold increase over baseline in CD8+ TEM cells and a 3.2- to 6.8-fold increase in TCM cells in each animal that received daily IL-15 at 15 μg/kg per day (Figure 3A-B). The proportion of CD8+ T cells in TN, TCM, and TEM fractions that expressed Ki-67 was increased during IL-15 administration, consistent with proliferation of these cells in vivo (Figure 3C-D; supplemental Figure 1C). Despite a similar increase in the fraction of Ki-67+ cells, the fold increase of CD8+ TEM cells in the blood was greater than TCM cells, which could be due to the migration of TCM cells from the blood pool to lymph nodes or other sites. The CD4+ TN, TCM, and TEM cells and the proportion of Ki-67+ TN, TCM, and TEM subsets also increased with daily IL-15, but to a lesser extent than CD8+ T cells (Figure 3E-H, supplemental Figure 1D). Collectively, the results demonstrated that daily subcutaneous administration of IL-15 increased the number and induced proliferation of circulating CD8+ and CD4+ memory T cells, but was associated with reversible toxicity.
Effect of daily IL-15 on absolute number and Ki-67 staining of CD8+ and CD4+ TN, TCM, and TEM subsets. (A-B) CD8+ TN, TCM, and TEM subsets. Samples of PBMCs from macaque 02269 (A) or macaque 05078 (B) at the indicated days before, during, and after the IL-15 treatment were stained with mAbs that bind to CD3, CD8, or CD95, and CCR7 to distinguish TN, TCM, and TEM subsets, and examined by flow cytometry. The data for each T-cell subset is shown as the fold increase of the absolute numbers per microliter of blood on the indicated day relative to the absolute cell numbers per microliter of the T-cell subset enumerated on the start of the treatment. (C-D) Expression of Ki-67 by CD8+ T-cell subsets. The data show the fraction of cells in percentage (%) within the CD8+ TN, TCM, and TEM subsets that express Ki-67 on the indicated days. (E-F) CD4+ TN, TCM, and TEM subsets. Samples of PBMCs from macaque 02269 (E) or macaque 05078 (F) at the indicated days before, during, and after the IL-15 treatment were stained with mAbs that bind to CD3, CD4, or CD95, and CCR7 to distinguish TN, TCM, and TEM subsets, and examined by flow cytometry. The data for each T-cell subset are shown as the fold increase of the absolute numbers per microliter of blood on the indicated day relative to the absolute cell numbers per microliter of the T-cell subset enumerated on the start of the treatment. (G-H) Expression of Ki-67 by CD4+ T-cell subsets. The data show the fraction of cells in percentage (%) within the CD4+ TN, TCM, and TEM subsets that express Ki-67 on the indicated days. → indicates the duration of the IL-15 treatment.
Effect of daily IL-15 on absolute number and Ki-67 staining of CD8+ and CD4+ TN, TCM, and TEM subsets. (A-B) CD8+ TN, TCM, and TEM subsets. Samples of PBMCs from macaque 02269 (A) or macaque 05078 (B) at the indicated days before, during, and after the IL-15 treatment were stained with mAbs that bind to CD3, CD8, or CD95, and CCR7 to distinguish TN, TCM, and TEM subsets, and examined by flow cytometry. The data for each T-cell subset is shown as the fold increase of the absolute numbers per microliter of blood on the indicated day relative to the absolute cell numbers per microliter of the T-cell subset enumerated on the start of the treatment. (C-D) Expression of Ki-67 by CD8+ T-cell subsets. The data show the fraction of cells in percentage (%) within the CD8+ TN, TCM, and TEM subsets that express Ki-67 on the indicated days. (E-F) CD4+ TN, TCM, and TEM subsets. Samples of PBMCs from macaque 02269 (E) or macaque 05078 (F) at the indicated days before, during, and after the IL-15 treatment were stained with mAbs that bind to CD3, CD4, or CD95, and CCR7 to distinguish TN, TCM, and TEM subsets, and examined by flow cytometry. The data for each T-cell subset are shown as the fold increase of the absolute numbers per microliter of blood on the indicated day relative to the absolute cell numbers per microliter of the T-cell subset enumerated on the start of the treatment. (G-H) Expression of Ki-67 by CD4+ T-cell subsets. The data show the fraction of cells in percentage (%) within the CD4+ TN, TCM, and TEM subsets that express Ki-67 on the indicated days. → indicates the duration of the IL-15 treatment.
Safety of intermittent administration of IL-15
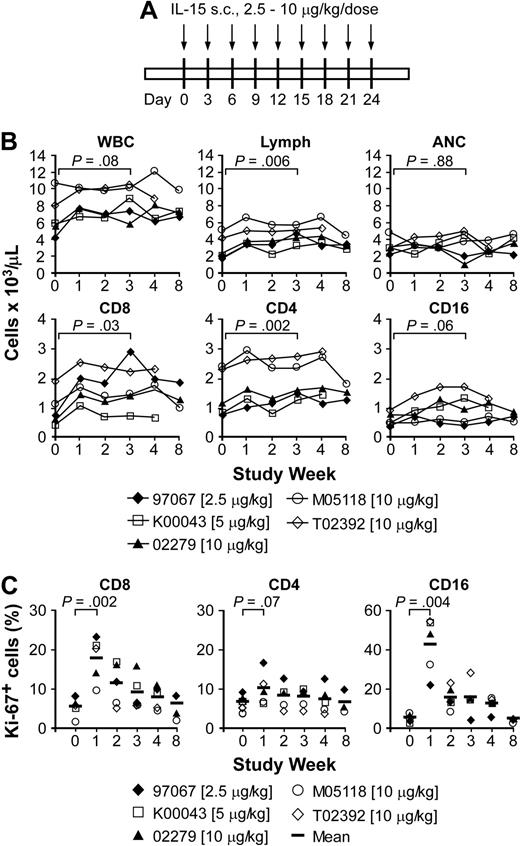
The reversible toxicities observed with daily IL-15 led us to examine whether less frequent dosing would enable prolonged administration without toxicity. Seven macaques received IL-15 given every 3 days at doses of 2.5 μg/kg (1 animal), 5 μg/kg (1 animal), or 10 μg/kg (5 animals) for 24 days (Figure 4A), and had measurements of plasma levels of IL-15 (Table 2). The IL-15 level 2 hours after injection ranged from 278.3 to 5765.8 pg/mL (Table 2). Analysis of the level 24 hours after injection of 10 μg/kg in 2 macaques revealed a persistent level (39.7-91.6 pg/mL; data not shown), but the trough plasma level obtained before the third or fifth injection was less than 10 pg/mL in all 7 animals (Table 2).
Effects of intermittent administration of IL-15 in immunocompetent macaques. (A) Schedule and dose of administration. Each  represents a single dose of IL-15 given every 3 days. (B) Absolute cell numbers of WBCs, lymphocytes, neutrophils (ANCs), as well as T-cell subsets, and NK cells per microliter of peripheral blood of macaques 97067, K00043, 02279, M05118, and T02392. Aliquots of the PBMCs were obtained from each of the macaques before, during, and after the IL-15 treatment, and stained with mAbs to CD3, CD4, CD8, and CD16. The data for CD3+CD8+, CD3+CD4+, and CD16+ cells are shown as the absolute cell number of each subset per microliter of peripheral blood at the indicated days. (C) Ki-67 expression. The percentage (%) of Ki-67+ cells within the CD3+CD8+, CD3+CD4+ T-cell, and CD16+ NK cell subsets is shown at the indicated days before, during, and after the IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.
represents a single dose of IL-15 given every 3 days. (B) Absolute cell numbers of WBCs, lymphocytes, neutrophils (ANCs), as well as T-cell subsets, and NK cells per microliter of peripheral blood of macaques 97067, K00043, 02279, M05118, and T02392. Aliquots of the PBMCs were obtained from each of the macaques before, during, and after the IL-15 treatment, and stained with mAbs to CD3, CD4, CD8, and CD16. The data for CD3+CD8+, CD3+CD4+, and CD16+ cells are shown as the absolute cell number of each subset per microliter of peripheral blood at the indicated days. (C) Ki-67 expression. The percentage (%) of Ki-67+ cells within the CD3+CD8+, CD3+CD4+ T-cell, and CD16+ NK cell subsets is shown at the indicated days before, during, and after the IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.
Effects of intermittent administration of IL-15 in immunocompetent macaques. (A) Schedule and dose of administration. Each  represents a single dose of IL-15 given every 3 days. (B) Absolute cell numbers of WBCs, lymphocytes, neutrophils (ANCs), as well as T-cell subsets, and NK cells per microliter of peripheral blood of macaques 97067, K00043, 02279, M05118, and T02392. Aliquots of the PBMCs were obtained from each of the macaques before, during, and after the IL-15 treatment, and stained with mAbs to CD3, CD4, CD8, and CD16. The data for CD3+CD8+, CD3+CD4+, and CD16+ cells are shown as the absolute cell number of each subset per microliter of peripheral blood at the indicated days. (C) Ki-67 expression. The percentage (%) of Ki-67+ cells within the CD3+CD8+, CD3+CD4+ T-cell, and CD16+ NK cell subsets is shown at the indicated days before, during, and after the IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.
represents a single dose of IL-15 given every 3 days. (B) Absolute cell numbers of WBCs, lymphocytes, neutrophils (ANCs), as well as T-cell subsets, and NK cells per microliter of peripheral blood of macaques 97067, K00043, 02279, M05118, and T02392. Aliquots of the PBMCs were obtained from each of the macaques before, during, and after the IL-15 treatment, and stained with mAbs to CD3, CD4, CD8, and CD16. The data for CD3+CD8+, CD3+CD4+, and CD16+ cells are shown as the absolute cell number of each subset per microliter of peripheral blood at the indicated days. (C) Ki-67 expression. The percentage (%) of Ki-67+ cells within the CD3+CD8+, CD3+CD4+ T-cell, and CD16+ NK cell subsets is shown at the indicated days before, during, and after the IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.
These results demonstrated that unlike daily subcutaneous injection, administration of IL-15 every 3 days allowed clearance of the cytokine before the next dose. Intermittent IL-15 treatment was well tolerated in all 7 animals without any alterations in clinical status. A BM biopsy in one of the animals at the end of IL-15 therapy exhibited normal morphology (supplemental Figure 2).
Immunologic effects of intermittent IL-15 administration
Five of the 7 animals receiving intermittent IL-15 had detailed analysis of blood counts and lymphocyte subsets before, during, and after IL-15 treatment. The data from these animals were pooled for subsequent analysis. Intermittent IL-15 resulted in only a modest increase in total WBC and neutrophil counts during the 4-week regimen at each dose level (Figure 4B). The changes in WBC and ANC were not statistically significant, but one of the macaques that received 10 μg/kg IL-15 had a transient decline in the ANC to .97 × 109/L (970/μL) by day 21 (Figure 4B). By contrast, the lymphocyte counts increased significantly during IL-15 treatment. Analysis of the phenotype of PBMCs showed that the increase in both the CD4+ and CD8+ T cells was statistically significant, whereas the increase in NK cell numbers was modest at all 3 dose levels (Figure 4B). The proportion of CD8+, CD4+, and CD16+ cells that expressed Ki-67 was also significantly increased above pretreatment values, but was substantially lower than that observed with daily administration of IL-15 at 15 μg/kg per day (Figure 4C). The subset of CD3+CD4−CD8− TCRγδ+ T cells was not boosted in the animals that received intermittent IL-15 (data not shown).
Examination of the number of CD8+ T cells in naive and memory subsets in the blood revealed a 0.8- to 1.8-fold increase in TN cells (P = .04), a 1.8- to 3.4-fold increase in CD8+ TCM cells (P = .002), and a 3.1- to 4-fold increase in CD8+ TEM cells (P < .001) by week 3 (Figure 5A). The proportion of Ki-67+ cells in the CD8+ TEM and TCM subsets peaked at days 6 to 8 after initiating IL-15 and this increase was statistically significant (Figure 5B-C). TN cells exhibited a very low proportion of Ki-67+ cells before IL-15, but also increased significantly (approximately 2-fold), consistent with the overall increase in TN cell numbers (Figure 5B-C).
Analysis of CD8+ TN, TCM, and TEM subsets during intermittent IL-15 administration. (A) Samples of PBMCs were obtained from macaques 97067, K00043, 02279, M05118, and T02392 before, during, and after the treatment with IL-15. Cells were stained with mAbs binding to CD3, CD8, or CD95, and CCR7 and analyzed by flow cytometry to distinguish TN (CD95lowCCR7+), TCM (CD95+CCR7+), and TEM (CD95+CCR7−) subsets. The fold increase of each subset at the indicated time of treatment is shown. The vertical bar represents the mean.  indicates the duration of the IL-15 treatment. The last posttreatment PBMC sample from K00043 was obtained on day 283. (B-C) Expression of Ki-67 by CD8+ T-cell subsets. (B) Representative data of macaque 02279. Gating of CD8+ TN, TCM, and TEM subsets stained for Ki-67 expression. PBMCs were stained with mAb specific for CD3, CD8, CD95, and CCR7, permeabilized, and stained with an antibody that binds to Ki-67, and analyzed by flow cytometry. Cells are gated on CD3+CD8+ T cells. The inset value in the upper right quadrant indicates the proportion of Ki-67+ T cells (in percentage). (C) The percentage (%) of Ki-67+ T cells within the CD8+ TN, TCM, and TEM subsets is shown at the indicated time of IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.
indicates the duration of the IL-15 treatment. The last posttreatment PBMC sample from K00043 was obtained on day 283. (B-C) Expression of Ki-67 by CD8+ T-cell subsets. (B) Representative data of macaque 02279. Gating of CD8+ TN, TCM, and TEM subsets stained for Ki-67 expression. PBMCs were stained with mAb specific for CD3, CD8, CD95, and CCR7, permeabilized, and stained with an antibody that binds to Ki-67, and analyzed by flow cytometry. Cells are gated on CD3+CD8+ T cells. The inset value in the upper right quadrant indicates the proportion of Ki-67+ T cells (in percentage). (C) The percentage (%) of Ki-67+ T cells within the CD8+ TN, TCM, and TEM subsets is shown at the indicated time of IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.  indicates the duration of the IL-15 treatment.
indicates the duration of the IL-15 treatment.
Analysis of CD8+ TN, TCM, and TEM subsets during intermittent IL-15 administration. (A) Samples of PBMCs were obtained from macaques 97067, K00043, 02279, M05118, and T02392 before, during, and after the treatment with IL-15. Cells were stained with mAbs binding to CD3, CD8, or CD95, and CCR7 and analyzed by flow cytometry to distinguish TN (CD95lowCCR7+), TCM (CD95+CCR7+), and TEM (CD95+CCR7−) subsets. The fold increase of each subset at the indicated time of treatment is shown. The vertical bar represents the mean.  indicates the duration of the IL-15 treatment. The last posttreatment PBMC sample from K00043 was obtained on day 283. (B-C) Expression of Ki-67 by CD8+ T-cell subsets. (B) Representative data of macaque 02279. Gating of CD8+ TN, TCM, and TEM subsets stained for Ki-67 expression. PBMCs were stained with mAb specific for CD3, CD8, CD95, and CCR7, permeabilized, and stained with an antibody that binds to Ki-67, and analyzed by flow cytometry. Cells are gated on CD3+CD8+ T cells. The inset value in the upper right quadrant indicates the proportion of Ki-67+ T cells (in percentage). (C) The percentage (%) of Ki-67+ T cells within the CD8+ TN, TCM, and TEM subsets is shown at the indicated time of IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.
indicates the duration of the IL-15 treatment. The last posttreatment PBMC sample from K00043 was obtained on day 283. (B-C) Expression of Ki-67 by CD8+ T-cell subsets. (B) Representative data of macaque 02279. Gating of CD8+ TN, TCM, and TEM subsets stained for Ki-67 expression. PBMCs were stained with mAb specific for CD3, CD8, CD95, and CCR7, permeabilized, and stained with an antibody that binds to Ki-67, and analyzed by flow cytometry. Cells are gated on CD3+CD8+ T cells. The inset value in the upper right quadrant indicates the proportion of Ki-67+ T cells (in percentage). (C) The percentage (%) of Ki-67+ T cells within the CD8+ TN, TCM, and TEM subsets is shown at the indicated time of IL-15 treatment of macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.  indicates the duration of the IL-15 treatment.
indicates the duration of the IL-15 treatment.
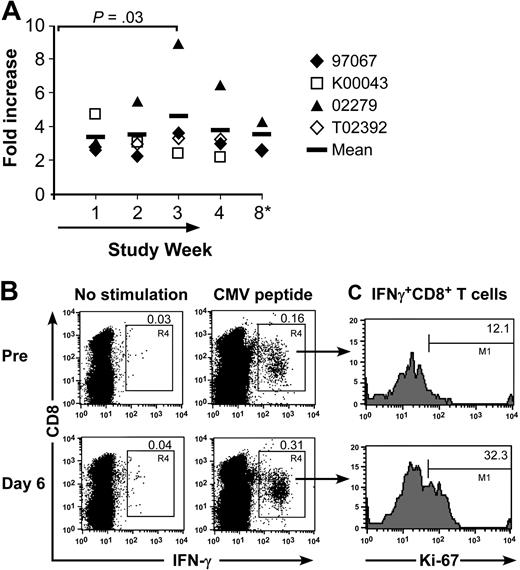
To address whether the overall increase in memory T cells would be reflected in a response to a specific pathogen, we analyzed the frequency of CD8+ memory T cells specific for defined CMV epitopes in 4 CMV-immune macaques before and after IL-15 administration using CFC.37 The absolute number of CMV-specific CD8+ T cells in the peripheral blood increased 3- to 9-fold (P = .03), which approximately paralleled the overall increase in memory T cells. The elevated number of circulating CMV-specific memory T cells gradually declined but remained in 2 of the animals above the pretreatment baseline value for at least 4 weeks after IL-15 was discontinued (Figure 6A). Costaining of CD8+ CMV-specific T cells in the CFC assay with Ki-67 demonstrated that IL-15 induced cycling in an increased fraction of the CMV-specific CD8+ T cells (Figure 6B-C).
Effect of IL-15 on circulating CMV-specific CD8+ memory T cells. (A) Samples of PBMCs were obtained from CMV-immune macaques 97067, K00043, 02279, and T02392 at the indicated time before, during, or after intermittent IL-15 administration. The samples were stimulated with the CMV peptide, and examined by CFC for the presence of IFN-γ–producing CMV-specific CD8+ T cells. Controls consisted of PBMCs cultured in medium alone to subtract background levels. The absolute number of CMV-specific CD8+ T cells per microliter of peripheral blood was calculated based on the frequency of T cells that produced IFN-γ after CMV-peptide stimulation at each time point and the absolute number of CD8+ T cells. The fold increase of the absolute number of CMV-specific CD8+ T cells per microliter of peripheral blood compared with the start of the treatment is shown. The vertical bar represents the mean.  indicates the duration of the IL-15 administration. *K00043 and T02392: no sample was available from week 8. (B-C) Representative data are shown for macaque K00043. PBMCs obtained before and on day 6 of the IL-15 treatment were stimulated with media alone or with CMV peptide, and examined by CFC for expression of IFN-γ and for Ki-67-expression, respectively. (B) The samples are gated on lymphocytes or (C) on peptide-stimulated CMV+ CD8+ IFN-γ+ T cells.
indicates the duration of the IL-15 administration. *K00043 and T02392: no sample was available from week 8. (B-C) Representative data are shown for macaque K00043. PBMCs obtained before and on day 6 of the IL-15 treatment were stimulated with media alone or with CMV peptide, and examined by CFC for expression of IFN-γ and for Ki-67-expression, respectively. (B) The samples are gated on lymphocytes or (C) on peptide-stimulated CMV+ CD8+ IFN-γ+ T cells.
Effect of IL-15 on circulating CMV-specific CD8+ memory T cells. (A) Samples of PBMCs were obtained from CMV-immune macaques 97067, K00043, 02279, and T02392 at the indicated time before, during, or after intermittent IL-15 administration. The samples were stimulated with the CMV peptide, and examined by CFC for the presence of IFN-γ–producing CMV-specific CD8+ T cells. Controls consisted of PBMCs cultured in medium alone to subtract background levels. The absolute number of CMV-specific CD8+ T cells per microliter of peripheral blood was calculated based on the frequency of T cells that produced IFN-γ after CMV-peptide stimulation at each time point and the absolute number of CD8+ T cells. The fold increase of the absolute number of CMV-specific CD8+ T cells per microliter of peripheral blood compared with the start of the treatment is shown. The vertical bar represents the mean.  indicates the duration of the IL-15 administration. *K00043 and T02392: no sample was available from week 8. (B-C) Representative data are shown for macaque K00043. PBMCs obtained before and on day 6 of the IL-15 treatment were stimulated with media alone or with CMV peptide, and examined by CFC for expression of IFN-γ and for Ki-67-expression, respectively. (B) The samples are gated on lymphocytes or (C) on peptide-stimulated CMV+ CD8+ IFN-γ+ T cells.
indicates the duration of the IL-15 administration. *K00043 and T02392: no sample was available from week 8. (B-C) Representative data are shown for macaque K00043. PBMCs obtained before and on day 6 of the IL-15 treatment were stimulated with media alone or with CMV peptide, and examined by CFC for expression of IFN-γ and for Ki-67-expression, respectively. (B) The samples are gated on lymphocytes or (C) on peptide-stimulated CMV+ CD8+ IFN-γ+ T cells.
IL-15 administration and CD4+CD25+Foxp3+ regulatory T cells
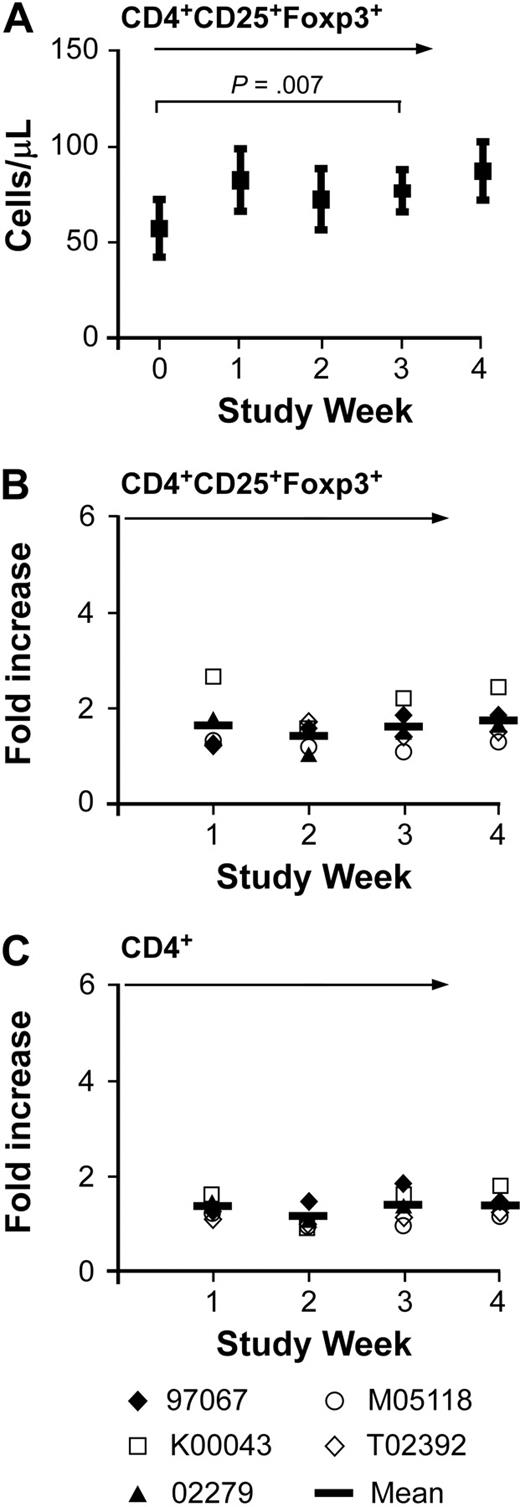
IL-2 is known to induce CD4+Foxp3+ regulatory T cells in vivo, and prior work has shown that high doses of IL-15 can induce CD4+CD25+Foxp3+ T cells in vitro and may also induce CD8+CD25+Foxp3+ T cells in vitro.7,44,45 Therefore, we examined whether CD4+CD25+Foxp3+ T regulatory cells were also increased in the peripheral blood in animals receiving intermittent IL-15 by staining PBMCs with mAbs for CD4, CD25, and Foxp3. The absolute number of CD4+CD25+Foxp3+ cells was 26 to 112/μL (mean: 57/μL) before therapy and 47 to 138/μL (mean: 87/μL) at week 4, after the IL-15 treatment (Figure 7A). The increase in cell number during the treatment was statistically significant, but there was no preferential increase in CD4+CD25+Foxp3+ T cells since the increase in CD4+CD25+Foxp3+ T cells was comparable with the overall increase of CD4+ T cells (Figure 7B-C). There were very few CD8+ T cells that stained positive for CD25 and Foxp3 before therapy (mean: 5/μL, range: 2-12/μL) and the mean absolute number at the end of IL-15 treatment was 9/μL (range: 4-26/μL; data not shown). Thus, intermittent subcutaneous administration of IL-15 resulted in only a small increment in the absolute numbers of circulating CD4+CD25+Foxp3+ or CD8+CD25+Foxp3+ cells, and did not preferentially expand these subsets of cells.
Absolute and relative numbers of Foxp3+ regulatory CD4+ T cells in immunocompetent macaques during IL-15 therapy. Samples of PBMCs obtained from macaques 97067, K00043, 02279, M05118, and T02392 were stained with mAbs binding to CD3, CD4, and CD25. The cells were then fixed and permeabilized, stained with an anti-Foxp3 antibody, and examined by flow cytometry. (A) The mean (± SEM) of the absolute cell number of CD4+Foxp3+ T cells per microliter of peripheral blood in the 5 macaques receiving intermittent IL-15 treatment at the indicated time is shown. (B-C) The fold increase of the absolute number of (B) CD4+CD25+Foxp3+ T cells per microliter or (C) CD4+ T cells per microliter in the peripheral blood compared with the start of the treatment is shown at the indicated time before, during, and after the respective intermittent IL-15 administration. Data are shown from macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.  indicates the duration of the IL-15 administration.
indicates the duration of the IL-15 administration.
Absolute and relative numbers of Foxp3+ regulatory CD4+ T cells in immunocompetent macaques during IL-15 therapy. Samples of PBMCs obtained from macaques 97067, K00043, 02279, M05118, and T02392 were stained with mAbs binding to CD3, CD4, and CD25. The cells were then fixed and permeabilized, stained with an anti-Foxp3 antibody, and examined by flow cytometry. (A) The mean (± SEM) of the absolute cell number of CD4+Foxp3+ T cells per microliter of peripheral blood in the 5 macaques receiving intermittent IL-15 treatment at the indicated time is shown. (B-C) The fold increase of the absolute number of (B) CD4+CD25+Foxp3+ T cells per microliter or (C) CD4+ T cells per microliter in the peripheral blood compared with the start of the treatment is shown at the indicated time before, during, and after the respective intermittent IL-15 administration. Data are shown from macaques 97067, K00043, 02279, M05118, and T02392. The vertical bar represents the mean.  indicates the duration of the IL-15 administration.
indicates the duration of the IL-15 administration.
Discussion
We examined the toxicity and immunologic effects of recombinant human IL-15 produced in mammalian cells and administered to nonhuman primates in a daily or intermittent dose schedule to provide insight into the potential clinical use of this cytokine. Daily IL-15 in doses of 5 to 15 μg/kg for 5 to 14 days caused distinct reversible side effects in 3 consecutive animals, including severe neutropenia, massive expansion of TCRγδ T cells, anemia and weight loss, and a generalized skin rash. The BM in the animal that developed neutropenia in our study was hypocellular consistent with lack of neutrophil production, which recovered rapidly when IL-15 was discontinued. Membrane-bound IL-15 has been reported to be overexpressed on BM stromal cells in aplastic anemia, suggesting a potential link to hematopoietic failure.46 Prior studies have also shown that CD34 progenitor cells express IL-15, which may have a function in regulating hematopoietic differentiation through autocrine/paracrine signaling.47 The induction of neutropenia by IL-15 requires additional study, but our data would suggest that the use of IL-15 to promote T-cell recovery early after hematopoietic stem cell transplantation or after chemotherapy when neutrophil counts are already low should be approached cautiously.
Toxicity from daily IL-15 administration was not described in a previous study in macaques in which daily doses of 10 μg/kg rhesus IL-15 were given to 3 macaques for up to 6 weeks.34 This apparent discrepancy may be explained by differences in the cytokine preparation used in the studies. Villinger et al administered recombinant rhesus IL-15 that was produced in bacteria to Macaca mulatta,34 whereas we administered recombinant human IL-15 prepared in mammalian cells to Macaca nemestrina. Thus, differences in glycosylation of the cytokines might affect biologic activity or clearance, or there may be species-specific susceptibility to toxicity.33 The report by Villinger et al did not include the blood counts of treated animals, and some changes in the ANC may have occurred.34 Finally, both studies administered daily IL-15 to a small number of animals, and larger cohorts of animals that receive different doses of IL-15 will be needed to provide a reliable estimate of the frequency of individual toxicities that occur after daily administration of IL-15, and to elucidate the mechanisms involved in the development of toxicity.
A notable observation in our study was that plasma IL-15 levels were persistently elevated in animals that received a daily subcutaneous injection of IL-15. In normal plasma, IL-15 levels are low or undetectable due in part to binding to IL-15Rα chains present on many cell types.12,39-43 The IL-15Rα/IL-15 complexes undergo endosomal internalization and are recycled to the cell surface,12,13 providing a persistent reservoir of IL-15 that can be presented in trans to neighboring IL-2Rβγc–bearing cells and be released into the environment.13,42 Thus, daily administration of IL-15 might result in accumulation of IL-15/IL-15Rα complexes that continuously release IL-15 locally and into the circulation. This putative mechanism for persistently high levels is consistent with a study in mice that were administered a single large bolus of IL-15, where serum levels declined with a rapid first phase followed by a slowly decaying second phase.42 The delayed clearance of IL-15 was mediated by IL-15Rα, since IL-15 was rapidly cleared in IL-15Rα−/− mice.42
We did not observe any toxicity when IL-15 was administered in doses of 2.5 to 10 μg/kg given every 3 days for 24 days to 7 consecutive animals. Similar peak IL-15 levels were obtained with intermittent dosing compared with daily dosing, but the cytokine was cleared before the next dose. A mild reversible decrement in the ANC was seen in one animal that received intermittent IL-15 at the 10-μg/kg per day dose level. Intermittent IL-15 had less dramatic but significant effects on CD8+, CD4+, and NK cell proliferation and absolute cell numbers than daily IL-15. Nevertheless, the absolute numbers of CD8 TCM and TEM cells in the blood were significantly increased and remained 2-fold above baseline for greater than 5 weeks after IL-15 was discontinued. In prior work, rhesus IL-15 has been administered to immunocompetent macaques alone or as a vaccine adjuvant and shown to increase the frequency of CD4+ and CD8+ T cells, respectively.34 Studies in SIV-infected macaques indicated a preferential expansion of circulating NK cells and the CD8+ TEM subset during IL-15 treatment, and an increased production and tissue emigration of CD4+ TEM cells.35,36 By contrast, little or no effects on the TN or TCM subsets were observed in SIV+ animals.35,36 In contrast to prior studies, we found that IL-15 induced an expansion of CD8+ TCM cells in the peripheral blood. The expansion of TCM cells that we observed might be due to the intact immune system in normal macaques compared with SIV-infected macaques and/or a dose effect.48
These studies demonstrate that IL-15 can be safely administered to nonhuman primates, and support the use of this cytokine in the clinic to augment endogenous T-cell and NK cell immunity. Prior reports in preclinical models demonstrated an important role of IL-15 alone or complexed to IL-15Rα in the induction and boosting of memory T-cell responses and suggested that IL-15 can serve as a mediator of CD4+ help in CD4+-deficient hosts.21,28 Our data showing a significant and durable increase in the frequency of memory T cells after IL-15 therapy suggest a potential clinical role for promoting functional memory T cells. However, the potential for adverse outcomes with IL-15 must be considered depending on the clinical circumstances. For example, IL-15 treatment increased viral replication and disease progression in SIV-infected macaques.49,50 An additional concern is the effect of IL-15 on immunosuppressive subsets of cells including CD4+CD25+Foxp3+ regulatory T cells. IL-15 in very high doses has been shown to increase CD4+Foxp3+ T cells in vitro, although these cells had weak suppressive activity.45 We observed only a small increase in circulating CD4+CD25+Foxp3+ T cells after in vivo administration of IL-15. Thus, in the doses studied here, IL-15 induces less expansion of CD4+CD25+Foxp3+ regulatory T cells than that observed with high-dose IL-2, which may be an important advantage for its use as an adjunct to tumor immunotherapy.7
Little is known regarding the use of IL-15 to support the survival of adoptively transferred T cells in vivo. A prior study in a murine model of melanoma using tumor-reactive T cells from TCR-transgenic mice showed that IL-15 either supplemented in culture, by exogenous administration, or constitutively expressed by transferred tumor-reactive T cells was more effective in tumor therapy compared with IL-2.30 We have previously shown that antigen-specific effector T (TE) cells derived from TCM precursors have an improved capacity to persist in vivo after adoptive transfer compared with effector T cells derived from TEM precursors.37 TCM-derived TE cells could be distinguished from TEM-derived TE cells by the ability of very low concentrations of IL-15 to rescue the cells from apoptosis after IL-2 withdrawal,37 suggesting a potential role for IL-15 in promoting the survival of transferred T cells in vivo. The intermittent dosing regimen of IL-15 that we used here is safe and provides peak plasma levels of IL-15 that are well above the concentration needed to support the in vitro survival of TE cells derived from TCM precursors.37 Studies to evaluate the intermittent administration of IL-15 with adoptively transferred T cells are in progress, and should provide important insights for the use of IL-15 for this indication in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Richard Lawler, Shared Resources Facility (FHCRC) for technical assistance with the ELISA assay and Ted Gooley, Clinical Research Division (FHCRC) for help with the statistical analysis. We thank Amgen for providing the human IL-15, and Jaclyn Bogue (WaNPRC) for assistance. We thank Louis Picker (Oregon Health & Science University) for the CMVpp65 peptide and helpful discussions.
This work was supported by National Institutes of Health grants CA114536 (S.R.R., C.B.), AI053193 (S.R.R.), and RR00166 (WaNPRC).
National Institutes of Health
Authorship
Contribution: C.B. designed and performed research, analyzed data, and wrote the paper; M.B., M.G., and C.E. performed research and analyzed the data; R.C.H. performed the histologic analysis; M.C.J. provided analytical reagents; and S.R.R. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Carolina Berger, Fred Hutchinson Cancer Research Center, Program in Immunology, D3-100, 1100 Fairview Ave N, PO Box 19024, Seattle, WA, 98109; e-mail: cberger@fhcrc.org.